Exposure Profiles for the Long-Term Use of Disinfectants and Cleaning Products and Asthma
- PMID: 39723603
- PMCID: PMC11969305
- DOI: 10.1111/all.16456
Exposure Profiles for the Long-Term Use of Disinfectants and Cleaning Products and Asthma
Abstract
Background: Using disinfectants and cleaning products (DCPs) at home and work is known to influence both the onset and course of asthma, but most epidemiological studies did not consider the multiplicity and correlations of exposures to DCPs. We aimed to identify exposure profiles for the long-term weekly use of DCPs by latent class analysis (LCA) and assess their associations with asthma.
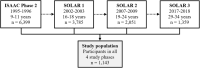
Methods: LCA was conducted on data from 1143 young adults initially recruited in the German centers of Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC) and followed up three times. In our LCA model, we included the use of cleaning sprays, disinfectant sprays, and nonspray disinfection methods, measured at ages 19-24 (first assessment) and 29-34 years (second assessment). Associations between identified exposure profiles and current as well as incident asthma/wheeze were evaluated by logistic regression.
Results: We identified five long-term exposure profiles to DCPs (latent classes): no weekly use of DCPs (55% of participants), use in first assessment (7%), use in second assessment (18%), persistent use (8%), and persistent cleaning sprays use (12%). Compared to "no weekly use," being in the "persistent use" profile was associated with both current asthma (OR = 1.68, 95% CI = [0.48-5.88]) and current wheeze (OR = 1.71, 95% CI = [0.75-3.90]). For incident asthma/wheeze, interval estimates were very wide.
Conclusions: Our study identified five distinct long-term exposure profiles to DCPs. Among those, only a persistent weekly use of multiple DCPs over time seemed to have an adverse effect on asthma. However, large confidence intervals indicate considerable uncertainty.
Keywords: asthma; disinfectants and cleaning products; exposure profiles; latent class analysis; sprays.
© 2024 The Author(s). Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
E.vM. reports grants from OM Pharma; consulting fees from OM Pharma, AstraZeneca; payment or honoraria from ALK‐Abello Arzneimittel GmbH, AstraZeneca, OM Pharma, and Abbott Laboratories; support for attending meetings and/or travel from Fabio Luigi Massimo Ricciardolo/Contatto S.r.l., Karl‐Landsteiner Private University for Health Sciences, Gordon Research Conferences, Arla, OM Pharma; participation on the BEAMS External Scientific Advisory Board (ESAB) and Abbott Allergy Risk Reduction Advisory Board. E.vM. has Patent No. PCT/EP2019/085016 (barn dust extract for the prevention and treatment of diseases) pending, royalties paid to ProtectImmun for patent EP2361632 (Specific environmental bacteria for the protection from and/or the treatment of allergic, chronic inflammatory and/or autoimmune disorders, granted on 19 March 2014), and patents EP1411977 (composition containing bacterial antigens used for the prophylaxis and the treatment of allergic diseases, granted on April 18, 2007), EP1637147 (stable dust extract for allergy protection, granted on December 10, 2008), and EP1964570 (pharmaceutical compound to protect against allergies and inflammatory diseases, granted on November 21, 2012) licensed to ProtectImmun. Patent EP21189353.2. 2021. von Mutius E, Rankl B, Bracher F, Müller C, Walker A, Hauck SM, Merl‐Pham J, and inventors; PROTEINS IDENTIFIED FROM BARN DUST EXTRACT FOR THE PREVENTION AND TREATMENT OF DISEASES. Patent PCT/US2021/016918. 2021. Martinez FD, Vercelli D, Snyder SA, von Mutius E, Pivniouk V, Marques dos Santos M, and inventors; THERAPEUTIC FRACTIONS AND PROTEINS FROM ASTHMA‐PROTECTIVE FARM DUST. Patent EP21189353.2. 2021. von Mutius E, Rankl B, Bracher F, Müller C, Walker A, Hauck SM, Merl‐Pham J, Adler H, Yildirim A.Ö., Sattler M, Santos Dias Mourao A, Borggräfe J, O'Connor P.D., Plettenburg O, and inventors; PROTEINS IDENTIFIED FROM BARN DUST EXTRACT FOR THE PREVENTION AND TREATMENT OF DISEASES.
C.V. reports a research grant from Boehringer Ingelheim; consulting fees from Sanofi Aventis; and payment or honoraria from Sanofi Aventis, AstraZeneca, and Novartis Pharma.
E.P.D.S., T.W., J. Gerlich, G.W., J. Genuneit, D.N., K.R., and F.F. declare no conflicts of interest.
Figures


Similar articles
-
Pulmonary rehabilitation versus usual care for adults with asthma.Cochrane Database Syst Rev. 2022 Aug 22;8(8):CD013485. doi: 10.1002/14651858.CD013485.pub2. Cochrane Database Syst Rev. 2022. PMID: 35993916 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Shared decision-making for people with asthma.Cochrane Database Syst Rev. 2017 Oct 3;10(10):CD012330. doi: 10.1002/14651858.CD012330.pub2. Cochrane Database Syst Rev. 2017. PMID: 28972652 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Chlorhexidine mouthrinse as an adjunctive treatment for gingival health.Cochrane Database Syst Rev. 2017 Mar 31;3(3):CD008676. doi: 10.1002/14651858.CD008676.pub2. Cochrane Database Syst Rev. 2017. PMID: 28362061 Free PMC article.
References
-
- 2024 GINA Main Report , “Global Initiative for Asthma,” accessed May 17, 2024, https://ginasthma.org/2024‐report/.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

