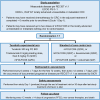
MOUNTAINEER-03 phase III study design: first-line mFOLFOX6 + tucatinib + trastuzumab for HER2+ metastatic colorectal cancer
- PMID: 39723627
- PMCID: PMC11792820
- DOI: 10.1080/14796694.2024.2441101
MOUNTAINEER-03 phase III study design: first-line mFOLFOX6 + tucatinib + trastuzumab for HER2+ metastatic colorectal cancer
Abstract
Patients diagnosed with metastatic colorectal cancer (mCRC) have a poor prognosis with survival ranging 2-3 years. The prevalence of human epidermal growth factor receptor 2 (HER2) amplification is approximately 3-4% in mCRC and increases up to 8% in patients with KRAS/NRAS/BRAF wild-type (WT) CRC tumors. Tucatinib is a highly selective HER2-directed tyrosine kinase inhibitor that, in combination with trastuzumab, has demonstrated clinically meaningful activity in patients with chemotherapy-refractory, HER2-positive (HER2+), RAS WT mCRC in the MOUNTAINEER trial. The MOUNTAINEER-03 phase III trial is designed to investigate the efficacy and safety of first-line tucatinib in combination with trastuzumab and modified FOLFOX6 (mFOLFOX6) versus standard of care (mFOLFOX6 plus bevacizumab or cetuximab) in patients with untreated HER2+, RAS WT locally advanced unresectable or mCRC. MOUNTAINEER-03 will include two arms of approximately 400 patients randomized 1:1 to either treatment arm. The primary endpoint is progression-free survival per RECIST v1.1 by blinded independent central review (BICR). Key secondary endpoints are overall survival and confirmed objective response rate (according to RECIST v1.1 per BICR). Safety assessments will include surveillance and recording of adverse events, physical examination findings, vital signs, cardiac assessments, Eastern Cooperative Oncology Group performance status, concomitant medications, and laboratory tests.Clinical trial registration: NCT05253651 (ClinicalTrials.gov).
Keywords: Bevacizumab; HER2; MOUNTAINEER-03; cetuximab; metastatic colorectal cancer; trastuzumab; tucatinib.
Plain language summary
Colorectal cancer is a common and leading cause of cancer deaths worldwide. Patients who have colorectal cancer that has spread to other parts of the body (metastasized) only live for 2 to 3 years after their diagnosis. Tucatinib is a medicine that has shown promising anti-tumor activity when it is given in combination with trastuzumab in patients with metastatic colorectal cancer that is of a certain type, called HER2-positive. HER2 is a protein that, when present in amounts above normal in cancer cells, can cause them to grow more and survive longer. The MOUNTAINEER-03 study is a trial in progress of patients with HER2-positive metastatic colorectal cancer. In this trial, researchers are investigating if tucatinib in combination with trastuzumab and chemotherapy increases the length of time patients live before their cancer grows or spreads (also called progression-free survival) and how long a person is alive after the start of treatment (also called overall survival) compared to standard of care treatment (chemotherapy combination). Researchers are also investigating whether this new tucatinib combination treatment makes the colorectal cancer shrink and stop spreading and if it is safe to use in patients with HER2-positive metastatic colorectal cancer. If the findings of the MOUNTAINEER-03 phase III trial are positive, the tucatinib combination treatment could be a new option for the first-line treatment of a patient population with a high unmet medical need. This paper describes the study design of the MOUNTAINEER-03 trial.
Conflict of interest statement
JS is a consultant or Advisory Role for AbbVie, Agenus, Astellas, AstraZeneca, Bayer, BeiGene, Daiichi-Sankyo, Eli Lilly, GSK, Johnson and Johnson, Jazz Pharmaceuticals, Merck, Natera, Pfizer, Roche/Genentech, Regeneron, Sanofi, Taiho, Takeda, Xilio Therapeutics; and has stocks in Triumvira Immunologics; and receives research funding or Contracted Research (Payment to Institution) from AbbVie, Amgen, AStar D3, Bayer, BeiGene, Curegenix, Daiichi-Sankyo, Eli Lilly, Erasca, GSK, Leap Therapeutics, Pfizer, Roche/Genentech, Seagen. T B-S receives research funding (to institution) from Agios, Arys, Arcus, Atreca, Boston Biomedical, Bayer, Eisai, Celgene, Lilly, Ipsen, Clovis, Seagen, Genentech, Novartis, Mirati, Merus, Abgenomics, Incyte, Pfizer, and BMS; and consulting fees (to institution) from Servier, Ipsen, Arcus, Pfizer, Seagen, Bayer, Genentech, Incyte, Eisai, Merus, Merck KGaA and Merck; and consulting fees (to self) from Stemline, AbbVie, Blueprint Medicines, Boehringer Ingelheim, Janssen, Daiichi Sankyo, Natera, TreosBio, Celularity, Caladrius Biosciences, Exact Science, Sobi, Beigene, Kanaph, AstraZeneca, Deciphera, Zai Labs, Exelixis, MJH Life Sciences, Aptitude Health, Illumina, Foundation Medicine, Sanofi, GSK, Xilio; IDMC/DSMB: The Valley Hospital, Fibrogen, Suzhou Kintor, AstraZeneca, Exelixis, Merck/Eisai, PanCan and 1Globe; Scientific Advisory Board: Imugene, Immuneering, Xilis, Replimune, Artiva and Sun Biopharma; and royalties from Uptodate; and inventions/patents for WO/2018/183488: HUMAN PD1 PEPTIDE VACCINES AND USES THEREOF – Licensed to Imugene and WO/2019/055687: METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER CACHEXIA – Licensed to Recursion. AC receives grants or contracts from GSK and Seagen; and consulting fees from Bayer, Merck, Pfizer, Roche, AbbVie, Seagen, and GSK; and patents planned, issued or pending for Neoadjuvant PD-1 blockade in solid tumors and HAI FUDR in DPD deficient patients with mCRC to liver; and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for ESMO and Fight CRC. VH receives honoraria from Merck, Amgen, Roche, Sanofi, Servier, Pfizer, Pierre-Fabre, AstraZeneca, BMS; MSD, Novartis, Boehringer Ingelheim, Celgene, SIRTEX, Seagen, GSK; and consulting fees or advisory role for Merck, Amgen, Roche, AstraZeneca, Celgene, Servier, Novartis, Pierre-Fabre, Halozyme, Merck Sharp & Dohme, Bristol Myers Squibb, GlaxoSmithKline, Janssen, Terumo, SIRTEX, OncoSil, NORDIC, Boehringer Ingelheim; and research funding (Payment to Institution) from Merck, Amgen, Roche, Celgene, Boehringer Ingelheim, Sirtex Medical, Shire; Stock: BioNTech. YN has an advisory role for Guardant Health Pte, Natera, Inc., Roche, Seagen, Premo Partners, Daiichi-Sankyo, Takeda, Exact Sciences Corporation, and Gilead Sciences; and receives a speakers’ bureau from Guardant Health Pte, Merck Sharp & Dohme K.K., Eisai, Zeria, Miyarisan, Merck Biopharma, Carenet, Hisamitsu, Taiho, Daiichi-Sankyo, Chugai, and Becton, Dickinson and Company, Guardant Health Japan Corp; and research funding from Seagen, Genomedia, Guardant Health AMEA, Guardant Health, Tempus Labs, Roche Diagnostics K.K., Daiichi-Sankyo, and Chugai. KR receives grants or contracts from AbbVie, AstraZeneca, Bayer, Daiichi Sankyo, Eisai, Genentech, Guardant Health, HiberCell, Innovent, Janssen, Merck, Seagen, UCB Biosciences, and Xencor; and consulting fees from AbbVie, AstraZeneca, Bayer, Daiichi Sankyo, Eisai, Genentech, and Seagen; and support for attending meetings and/or travel from AstraZeneca, Bayer, Daiichi Sankyo, Eisai, Genentech, Jazz Pharma, Seagen, and Sanofi. SS is on the advisory board for Agenus, AstraZeneca, Bayer, Bristol Myers Squibb, CheckmAb, Daiichi-Sankyo, GlaxoSmithKline, Merck Sharp & Dohme, Merck, Novartis, Pierre-Fabre, Seagen, and T-One Therapeutics. JT is a consultant or advisory role or scientific consultancy role for Alentis, AstraZeneca, Aveo Oncology, Boehringer Ingelheim, Cardiff Oncology, CARsgen Therapeutics, Chugai, Daiichi-Sankyo, F. Hoffmann-La Roche, Genentech, hC Bioscience Inc, Ikena Oncology, Immodulon Therapeutics, Inspirna, IQVIA, Lilly, Menarini, Merck Serono, Merus, Mirati, Merck Sharp & Dohme, NeoPhore, Novartis, Ona Therapeutics, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Sanofi, Scandion Oncology, Scorpion Therapeutics, Seagen, Servier, Sotio Biotech, Taiho, Takeda, and Tolremo Therapeutics; Stock Ownership: 1TRIALSP, Alentis Therapeutics, Oniria Therapeutics, Pangaea Oncology. EvC participates on advisory boards for AbbVie, Agenus, ALX, Amgen, Arcus Biosciences, Astellas, AstraZeneca, Bayer, Beigene, Bexon Clinical, BioNtech, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi, Debiopharma, Elmedix, Eisai, Galapagos, GSK, Hookipa Biotech, Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Mirati, Novartis, Nordic, Pierre Fabre, Pfizer, Roche, Seagen, Servier, Simcere, Takeda, Taiho, and Terumo. TY receives honoraria for Chugai, Takeda, Merck, Bayer Yakuhin, Ono, and Merck Sharp & Dohme K.K; and consulting fees for Sumitomo Corp.; and research grants from Amgen, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi-Sankyo, Eisai, FALCO biosystems, Genomedia, Medical & Biological Laboratories, Merus N.V., Molecular Health GmbH, MSD, Nippon Boehringer Ingelheim, Ono, Pfizer Japan, Roche Diagnostics, Sanofi, Sysmex, Taiho, and Takeda. JR is an employee at Pfizer Inc. XG is an employee at Pfizer Inc. TA is on the advisory board and receives consulting fees from AbbVie, Astellas, Aptitude Health, Bristol Myers Squibb, Gritstone Oncology, GlaxoSmithKline, Merck & Co. Inc, Nordic Oncology, Seagen, Servier, Takeda; and honoraria from Bristol Myers Squibb, GlaxoSmithKline, Merck & Co. Inc, Merck Serono, Roche, Sanofi, Seagen, and Servier; DMC: Inspirna; and support for meetings from Bristol Myers Squibb and Merck. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing support was provided by Jessica Men, PharmD, and Anastasija Pesevska, PharmD, and editorial support was provided by Melissa Ward, BA, all of Scion (a division of Prime, London, UK), supported by Seagen Inc., which was acquired by Pfizer in Dec. 2023, in accordance with Good Publication Practice guidelines (
Figures
References
-
- World Health Organization . Cancer. 2022. [cited 2024 Mar 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
• Global estimates of incidence and mortality of patients with cancer.
-
- Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011–2019. - PubMed
-
- Cervantes A, Adam R, Rosello S, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(1):10–32. - PubMed
-
•• ESMO clinical guidelines for metastatic colorectal cancer.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous