Distinct Gut Microbiota Profiles in Normal Weight Obesity and Their Association With Cardiometabolic Diseases: Results From Two Independent Cohort Studies
- PMID: 39723699
- PMCID: PMC11670180
- DOI: 10.1002/jcsm.13644
Distinct Gut Microbiota Profiles in Normal Weight Obesity and Their Association With Cardiometabolic Diseases: Results From Two Independent Cohort Studies
Abstract
Background: Normal weight obesity (NWO) is characterized by excess body fat in individuals with normal body mass index (BMI). This study aimed to investigate gut microbiota alterations in NWO and their potential associations with cardiometabolic diseases (CMD) risk in two independent cohorts.
Methods: Our NWO-CMD mortality analysis included 168 099 adults with normal BMI from two large open-access databases, while our NWO-gut microbiota study involved 5467 adults with normal BMI from two independent cohorts: the WELL-China cohort and the Lanxi cohort. NWO was defined as having a normal BMI (18.5-23.9 kg/m2) but an excess per cent body fat (PBF, ≥ 25% in men and ≥ 35% in women). Normal weight lean was defined as having a normal BMI and normal PBF. The 16S rRNA gene sequencing method was used to analyse gut microbiota data.
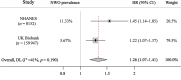
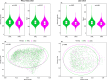
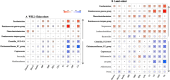
Results: The study comprised 3620 (64.0% female, median age 58 years) and 1847 (64.3% female, median age 56 years) participants from the WELL-China and Lanxi cohorts. In our meta-analysis, NWO is associated with 26% (95% CI: 1.07-1.41) higher risk of CMD mortality. Gut microbial analyses indicated that the NWO group exhibited reduced levels of observed species (p = 0.009 and p = 0.013) and Chao 1 index (p = 0.002 and p = 0.002) and altered gut microbial compositions (p = 0.009 and p < 0.001) compared with the NWL group. Seven genera were consistently observed to be associated with NWO in both two cohorts (all Q < 0.25). Among them, five (Fusobacterium, Ruminococcus gnavus group, Ruminococcus torques group, Coprococcus and Christensenellaceae_R7_group) have been previously linked to obesity, while the other two (Phascolarctobacterium and Clostridia_UCG-014) were minimally reported. We also found statistically significant differences in the microbial composition between the NWO group and the obesity group (p = 0.001 and p = 0.001). Furthermore, the NWO-related gut microbiome was associated with an elevated risk of hypertension, dyslipidaemia and metabolic syndrome, the corresponding HR (95% CIs) were 1.11 (1.01-1.22), 1.19 (1.10-1.29) and 1.17 (1.05-1.30) in the WELL-China cohort and 1.14 (1.02-1.27), 1.15 (1.02-1.29) and 1.16 (1.02-1.32) in the Lanxi cohort.
Conclusions: These two large cohorts provided reliable evidence that gut microbiota alterations in NWO resemble those found in obesity, yet also display unique aspects. This distinct microbiota profile may contribute to heightened cardiometabolic risks in adults with normal BMI.
Keywords: Cardiometabolic diseases; Gut microbiota; Independent cohort; Normal weight obesity.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Lv Y., Qin X., Jia H., Chen S., Sun W., and Wang X., “The Association Between Gut Microbiota Composition and BMI in Chinese Male College Students, as Analysed by Next‐Generation Sequencing,” British Journal of Nutrition 122 (2019): 986–995. - PubMed
-
- Mahase E., “Stop Using Body Mass Index as Measure of Health, say MPs,” BMJ 373 (2021): n941. - PubMed
-
- Oliveros E., Somers V. K., Sochor O., Goel K., and Lopez‐Jimenez F., “The Concept of Normal Weight Obesity,” Progress in Cardiovascular Diseases 56 (2014): 426–433. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

