Succinate Regulates Exercise-Induced Muscle Remodelling by Boosting Satellite Cell Differentiation Through Succinate Receptor 1
- PMID: 39723719
- PMCID: PMC11670172
- DOI: 10.1002/jcsm.13670
Succinate Regulates Exercise-Induced Muscle Remodelling by Boosting Satellite Cell Differentiation Through Succinate Receptor 1
Abstract
Background: Skeletal muscle remodelling can cause clinically important changes in muscle phenotypes. Satellite cells (SCs) myogenic potential underlies the maintenance of muscle plasticity. Accumulating evidence shows the importance of succinate in muscle metabolism and function. However, whether succinate can affect SC function and subsequently coordinate muscle remodelling to exercise remains unexplored.
Methods: A mouse model of high-intensity interval training (HIIT) was used to investigate the effects of succinate on muscle remodelling and SC function by exercise capacity test and biochemical methods. Mice with succinate receptor 1 (SUCNR1)-specific knockout in SCs were generated as an in vivo model to explore the underlying mechanisms. RNA sequencing of isolated SCs was performed to identify molecular changes responding to succinate-SUCNR1 signalling. The effects of identified key molecules on the myogenic capacity of SCs were investigated using gain- and loss-of-function assays in vitro. To support the translational application, the clinical efficacy of succinate was explored in muscle-wasting mice.
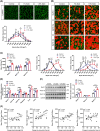
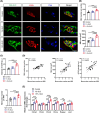
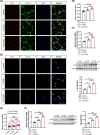
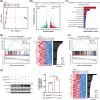
Results: After 21 days of HIIT, mice supplemented with 1.5% succinate exhibited striking gains in grip strength (+0.38 ± 0.04 vs. 0.26 ± 0.03 N, p < 0.001) and endurance (+276.70 ± 55.80 vs. 201.70 ± 45.31 s, p < 0.05), accompanied by enhanced muscle hypertrophy and neuromuscular junction regeneration (p < 0.001). The myogenic capacity of SCs was significantly increased in gastrocnemius muscle of mice supplemented with 1% and 1.5% succinate (+16.48% vs. control, p = 0.008; +47.25% vs. control, p < 0.001, respectively). SUCNR1-specific deletion in SCs abolished the modulatory influence of succinate on muscle adaptation in response to exercise, revealing that SCs respond to succinate-SUCNR1 signalling, thereby facilitating muscle remodelling. SUCNR1 signalling markedly upregulated genes associated with stem cell differentiation and phosphorylation pathways within SCs, of which p38α mitogen-activated protein kinase (MAPK; fold change = 6.7, p < 0.001) and protein kinase C eta (PKCη; fold change = 12.5, p < 0.001) expressions were the most enriched, respectively. Mechanistically, succinate enhanced the myogenic capacity of isolated SCs by activating the SUCNR1-PKCη-p38α MAPK pathway. Finally, succinate promoted SC differentiation (1.5-fold, p < 0.001), ameliorating dexamethasone-induced muscle atrophy in mice (p < 0.001).
Conclusions: Our findings reveal a novel function of succinate in enhancing SC myogenic capacity via SUCNR1, leading to enhanced muscle adaptation in response to exercise. These findings provide new insights for developing pharmacological strategies to overcome muscle atrophy-related diseases.
Keywords: PKCη; SUCNR1; muscle remodelling; satellite cells; succinate.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
pH-Gated Succinate Secretion Regulates Muscle Remodeling in Response to Exercise.Cell. 2020 Oct 1;183(1):62-75.e17. doi: 10.1016/j.cell.2020.08.039. Epub 2020 Sep 17. Cell. 2020. PMID: 32946811 Free PMC article.
-
Cell selectivity in succinate receptor SUCNR1/GPR91 signaling in skeletal muscle.Am J Physiol Endocrinol Metab. 2023 Apr 1;324(4):E289-E298. doi: 10.1152/ajpendo.00009.2023. Epub 2023 Feb 22. Am J Physiol Endocrinol Metab. 2023. PMID: 36812387
-
Acute Succinate Administration Increases Oxidative Phosphorylation and Skeletal Muscle Explosive Strength via SUCNR1.Front Vet Sci. 2022 Jan 14;8:808863. doi: 10.3389/fvets.2021.808863. eCollection 2021. Front Vet Sci. 2022. PMID: 35097053 Free PMC article.
-
Control of muscle satellite cell function by specific exercise-induced cytokines and their applications in muscle maintenance.J Cachexia Sarcopenia Muscle. 2024 Apr;15(2):466-476. doi: 10.1002/jcsm.13440. Epub 2024 Feb 20. J Cachexia Sarcopenia Muscle. 2024. PMID: 38375571 Free PMC article. Review.
-
The quasi-parallel lives of satellite cells and atrophying muscle.Front Aging Neurosci. 2015 Jul 22;7:140. doi: 10.3389/fnagi.2015.00140. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26257645 Free PMC article. Review.
Cited by
-
From inflammation to healing: the crucial role of GPR91 activation and SDH inhibition in chronic diabetic wound recovery.Stem Cell Res Ther. 2025 Jul 23;16(1):399. doi: 10.1186/s13287-025-04480-6. Stem Cell Res Ther. 2025. PMID: 40702577 Free PMC article.
-
Physical Activity, Exerkines, and Their Role in Cancer Cachexia.Int J Mol Sci. 2025 Aug 19;26(16):8011. doi: 10.3390/ijms26168011. Int J Mol Sci. 2025. PMID: 40869331 Free PMC article. Review.
References
-
- Folland J. P. and Williams A. G., “The Adaptations to Strength Training: Morphological and Neurological Contributions to Increased Strength,” Sports Medicine 37 (2007): 145–168. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

