A Copper-Binding Peptide with Therapeutic Potential against Alzheimer's Disease: From the Blood-Brain Barrier to Metal Competition
- PMID: 39723808
- PMCID: PMC11741003
- DOI: 10.1021/acschemneuro.4c00796
A Copper-Binding Peptide with Therapeutic Potential against Alzheimer's Disease: From the Blood-Brain Barrier to Metal Competition
Abstract
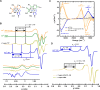
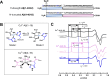
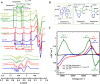
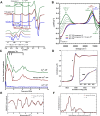
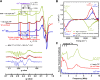
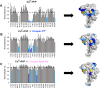
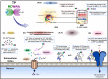
Alzheimer's disease (AD) is the most common form of dementia worldwide. AD brains are characterized by the accumulation of amyloid-β peptides (Aβ) that bind Cu2+ and have been associated with several neurotoxic mechanisms. Although the use of copper chelators to prevent the formation of Cu2+-Aβ complexes has been proposed as a therapeutic strategy, recent studies show that copper is an important neuromodulator that is essential for a neuroprotective mechanism mediated by Cu2+ binding to the cellular prion protein (PrPC). Therefore, in addition to metal selectivity and blood-brain barrier (BBB) permeability, an emerging challenge for copper chelators is to prevent the formation of neurotoxic Cu2+-Aβ species without perturbing the neuroprotective Cu2+-PrPC interaction. Previously, we reported the design of a tetrapeptide (TP) that withdraws Cu2+ from Aβ(1-16) and impacts the Cu2+-induced aggregation of Aβ(1-40). In this study, we improved the drug-like properties of TP in a BBB model, evaluated the metal selectivity of the optimized peptide (TP*), and tested its effect on Cu2+ coordination to PrPC and proteins involved in copper trafficking, such as copper transporter 1 and albumin. Our results show that changing the stereochemistry of the first residue prevents TP degradation in the BBB model and coadministration of TP with a peptide that increases BBB permeability allows its passage through the BBB model. TP* is highly selective toward Cu2+ in the presence of Zn2+ ions, transfers Cu2+ to copper-trafficking proteins, and forms a ternary TP*-Cu2+-PrP species that does not perturb the physiological conformation of PrP and displays only a minor impact in the neuroprotective Cu2+-dependent interaction of PrPC with the N-methyl-d-aspartate receptor. Overall, these results show that TP* displays desirable features for a copper chelator with therapeutic potential against AD. Moreover, this is the first study that explores the effect of a Cu2+ chelator with therapeutic potential for AD on Cu2+ coordination to PrPC (an emerging key player in AD pathology), integrating recent knowledge about metalloproteins involved in AD with the design of copper chelators against AD.
Keywords: Alzheimer′s disease; NMDA receptor; chelation therapy; copper; prion protein.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Organization, W. H. Dementia. 2019. https://www.who.int/news-room/fact-sheets/detail/dementia. (accessed July 4, 2024).
-
- Organization, W. H. Causes of Death. 2019. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. (accessed July 4, 2024).
-
- Wang H.; Wang M.; Wang B.; Li M.; Chen H.; Yu X.; Yang K.; Chai Z.; Zhao Y.; Feng W. Immunogold labeling and X-ray fluorescence microscopy reveal enrichment ratios of Cu and Zn, metabolism of APP and amyloid-beta plaque formation in a mouse model of Alzheimer’s disease. Metallomics 2012, 4 (10), 1113–1118. 10.1039/c2mt20056b. - DOI - PubMed
-
From NLM Medline
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

