Regulation mechanism of the long-chain n-alkane monooxygenase gene almA in Acinetobacter venetianus RAG-1
- PMID: 39723816
- PMCID: PMC11784139
- DOI: 10.1128/aem.02050-24
Regulation mechanism of the long-chain n-alkane monooxygenase gene almA in Acinetobacter venetianus RAG-1
Abstract
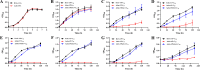
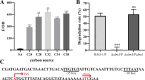
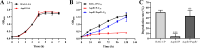
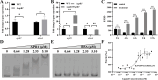
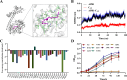
As toxic pollutants, n-alkanes are pervasively distributed in most environmental matrices. Although the alkane monooxygenase AlmA plays a critical role in the metabolic pathway of solid long-chain n-alkanes (≥C20) that are extremely difficult to degrade, the mechanism regulating this process remains unclear. Here, we characterized the function of AlmA in Acinetobacter venetianus RAG-1, which was mainly involved in the degradation of long-chain n-alkanes (C26-C38), among which, n-C32 induced the almA promoter activity most. APR1 (
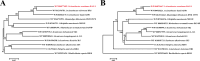
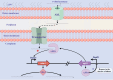
Importance: The extreme hydrophobicity of long-chain n-alkanes ({greater than or equal to}C20) presents a significant challenge to their degradation in natural environments. It is, therefore, imperative to elucidate the regulatory mechanisms of the metabolic pathways of long-chain n-alkanes, which will be of great significance for the future application of hydrocarbon-degrading bacteria to treat oil spills. However, the majority of current studies have focused on the regulatory mechanisms of short- and medium-chain n-alkanes, with long-chain n-alkanes receiving comparatively little attention. In this study, we identified APR1, a transcriptional regulator of the alkane monooxygenase AlmA in Acinetobacter venetianus RAG-1, and characterized its function and regulatory mechanism. In the presence of ligand n-C32, APR1 could directly activate the transcription of almA, which was involved in the n-C32 metabolism. The amino acid residue unique to the C-terminal DNA-binding domain of AraC/XylS type n-alkanes transcription regulators was also identified. These findings further improved our understanding of the long-chain n-alkanes degradation mechanism, which is important for the management of petroleum pollution.
Keywords: APR1; Acinetobacter venetianus RAG-1; AlmA; AraC/XylS family transcriptional regulator; long-chain n-alkanes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
AlmA involved in the long-chain n-alkane degradation pathway in Acinetobacter baylyi ADP1 is a Baeyer-Villiger monooxygenase.Appl Environ Microbiol. 2024 Jan 24;90(1):e0162523. doi: 10.1128/aem.01625-23. Epub 2024 Jan 3. Appl Environ Microbiol. 2024. PMID: 38168668 Free PMC article.
-
A Novel FadL Homolog, AltL, Mediates Transport of Long-Chain Alkanes and Fatty Acids in Acinetobacter venetianus RAG-1.Appl Environ Microbiol. 2022 Oct 26;88(20):e0129422. doi: 10.1128/aem.01294-22. Epub 2022 Sep 28. Appl Environ Microbiol. 2022. PMID: 36169310 Free PMC article.
-
Enhanced degradation of different crude oils by defined engineered consortia of Acinetobacter venetianus RAG-1 mutants based on their alkane metabolism.Bioresour Technol. 2021 May;327:124787. doi: 10.1016/j.biortech.2021.124787. Epub 2021 Feb 1. Bioresour Technol. 2021. PMID: 33556770
-
The structure-function relationship of bacterial transcriptional regulators as a target for enhanced biodegradation of aromatic hydrocarbons.Microbiol Res. 2022 Sep;262:127087. doi: 10.1016/j.micres.2022.127087. Epub 2022 Jun 7. Microbiol Res. 2022. PMID: 35717889 Review.
-
Arac/XylS family of transcriptional regulators.Microbiol Mol Biol Rev. 1997 Dec;61(4):393-410. doi: 10.1128/mmbr.61.4.393-410.1997. Microbiol Mol Biol Rev. 1997. PMID: 9409145 Free PMC article. Review.
Cited by
-
Alkane degradation mechanism of Mixta calida HXX308 isolated from sediment of the Mariana Trench.Front Microbiol. 2025 Apr 28;16:1579612. doi: 10.3389/fmicb.2025.1579612. eCollection 2025. Front Microbiol. 2025. PMID: 40356637 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

