Ultrastructural imaging biomarkers in diabetic macular edema: A major review
- PMID: 39723865
- PMCID: PMC11834929
- DOI: 10.4103/IJO.IJO_878_24
Ultrastructural imaging biomarkers in diabetic macular edema: A major review
Abstract
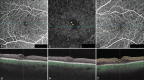
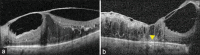
Diabetic macular edema (DME) is a vision-threatening complication of diabetic retinopathy and causes significant morbidity in patients. Anti-vascular endothelial growth factor (VEGF) agents are the mainstay of treatment for DME, with steroid implants being used for the treatment of anti-VEGF resistant eyes. Over the years, several classification systems have been devised to describe the patterns of DME using optical coherence tomography (OCT). With the advent of effective treatments, it has become imperative that imaging cues are not merely used for classifying the disease but also as biomarkers for prognostication of disease activity and treatment response. In this aspect, newer imaging findings such as hyperreflective dots, photoreceptor integrity, and disorganization of retinal inner layers have been characterized in detail by several authors. Macular perfusion analysis using OCT angiography is the latest in the armamentarium for imaging DME. In this narrative review, we have summarized all relevant literature related to the ultrastructural imaging-based biomarkers of DME and their correlation to treatment.
Copyright © 2024 Indian Journal of Ophthalmology.
Conflict of interest statement
There are no conflicts of interest.
Figures






Similar articles
-
Optical Coherence Tomography Angiography of DME and Its Association with Anti-VEGF Treatment Response.Ophthalmology. 2016 Nov;123(11):2368-2375. doi: 10.1016/j.ophtha.2016.07.010. Epub 2016 Sep 6. Ophthalmology. 2016. PMID: 27613201
-
Review on Recent Trials Evaluating the Effect of Intravitreal Injections of Anti-VEGF Agents on the Macular Perfusion of Diabetic Patients with Diabetic Macular Edema.Rev Recent Clin Trials. 2020;15(3):188-198. doi: 10.2174/1574887115666200519073704. Rev Recent Clin Trials. 2020. PMID: 32427087 Free PMC article.
-
Choroidal Variations in Diabetic Macular Edema: Fluorescein Angiography and Optical Coherence Tomography.Curr Eye Res. 2018 Jan;43(1):102-108. doi: 10.1080/02713683.2017.1370115. Epub 2017 Sep 22. Curr Eye Res. 2018. PMID: 28937826
-
Dril Influences Short-term Visual Outcome after Intravitreal Corticosteroid Injection for Refractory Diabetic Macular Edema.Curr Eye Res. 2021 Sep;46(9):1378-1386. doi: 10.1080/02713683.2021.1878540. Epub 2021 Jan 31. Curr Eye Res. 2021. PMID: 33463388
-
Pathological Neurovascular Unit Mapping onto Multimodal Imaging in Diabetic Macular Edema.Medicina (Kaunas). 2023 May 7;59(5):896. doi: 10.3390/medicina59050896. Medicina (Kaunas). 2023. PMID: 37241128 Free PMC article. Review.
Cited by
-
A Study of Visual Outcomes and Spectral Domain Optical Coherence Tomography (SD-OCT) Biomarker Changes in Patients Treated With Ranibizumab for Diabetic Macular Edema in a Tertiary Hospital.Cureus. 2025 Apr 18;17(4):e82520. doi: 10.7759/cureus.82520. eCollection 2025 Apr. Cureus. 2025. PMID: 40385737 Free PMC article.
-
A machine learning model for predicting anatomical response to Anti-VEGF therapy in diabetic macular edema.Front Cell Dev Biol. 2025 May 30;13:1603958. doi: 10.3389/fcell.2025.1603958. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40519258 Free PMC article.
-
Erratum: Ultrastructural imaging biomarkers in diabetic macular edema: A major review.Indian J Ophthalmol. 2025 Feb 1;73(2):309. doi: 10.4103/IJO.IJO_107_25. Epub 2025 Jan 24. Indian J Ophthalmol. 2025. PMID: 39853148 Free PMC article. No abstract available.
References
-
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, et al. Diabetic retinopathy. Diabetes Care. 1998;21:143–56. - PubMed
-
- Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early treatment diabetic retinopathy study report no. 3. The early treatment diabetic retinopathy study research group. Int Ophthalmol Clin. 1987;27:254–64. - PubMed
-
- Wilkinson CP, Ferris FL, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

