Glucose-dependent insulinotropic polypeptide receptor signaling alleviates gut inflammation in mice
- PMID: 39723966
- PMCID: PMC11948578
- DOI: 10.1172/jci.insight.174825
Glucose-dependent insulinotropic polypeptide receptor signaling alleviates gut inflammation in mice
Abstract
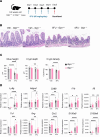
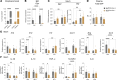
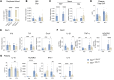
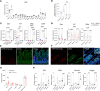
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) are gut-derived peptide hormones that potentiate glucose-dependent insulin secretion. The clinical development of GIP receptor-GLP-1 receptor (GIPR-GLP-1R) multiagonists exemplified by tirzepatide and emerging GIPR antagonist-GLP-1R agonist therapeutics such as maritide is increasing interest in the extrapancreatic actions of incretin therapies. Both GLP-1 and GIP modulate inflammation, with GLP-1 also acting locally to alleviate gut inflammation in part through antiinflammatory actions on GLP-1R+ intestinal intraepithelial lymphocytes. In contrast, whether GIP modulates gut inflammation is not known. Here, using gain- and loss-of-function studies, we show that GIP alleviates 5-fluorouracil-induced (5FU-induced) gut inflammation, whereas genetic deletion of Gipr exacerbates the proinflammatory response to 5FU in the murine small bowel (SB). Bone marrow (BM) transplant studies demonstrated that BM-derived Gipr-expressing cells suppress 5FU-induced gut inflammation in the context of global Gipr deficiency. Within the gut, Gipr was localized to nonimmune cells, specifically stromal CD146+ cells. Hence, the extrapancreatic actions of GIPR signaling extend to the attenuation of gut inflammation, findings with potential translational relevance for clinical strategies modulating GIPR action in people with type 2 diabetes or obesity.
Keywords: Diabetes; Endocrinology.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

