Resuscitation in Paediatric Septic Shock Using Vitamin C and Hydrocortisone (RESPOND): The RESPOND Randomized Controlled Trial Protocol
- PMID: 39724024
- PMCID: PMC11878590
- DOI: 10.1097/PCC.0000000000003674
Resuscitation in Paediatric Septic Shock Using Vitamin C and Hydrocortisone (RESPOND): The RESPOND Randomized Controlled Trial Protocol
Abstract
Objectives: Pediatric sepsis results in significant morbidity and mortality worldwide. There is an urgent need to investigate adjunctive therapies that can be administered early. We hypothesize that using vitamin C combined with hydrocortisone increases survival free of inotropes/vasopressors support until day 7 compared with standard care. Here we describe the Resuscitation in Paediatric Septic Shock using Vitamin C and Hydrocortisone (RESPOND) trial protocol, which aims to address this hypothesis.
Design: Randomized, open label, controlled, parallel-group, three-arm trial with integrated economic evaluation.
Setting: Nine Australia and New Zealand PICUs, with interest from additional international sites.
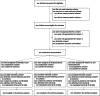
Patients: Children between 7 days and younger than 18 years old who are treated for suspected or confirmed sepsis and receiving inotropes/vasopressors for greater than 1 hour.
Interventions: IV vitamin C (100 mg/kg [maximum 5 g] every 6 hr) and hydrocortisone (1 mg/kg [maximum 50 mg] every 6 hr), or IV hydrocortisone alone (1 mg/kg [maximum 50 mg] every 6 hr) or standard care.
Measurements and main results: Three hundred eighty-four children will be randomly assigned to receive the interventions, or standard care in a 1:1:1 ratio with stratification by steroid administration pre-randomization and hospital site. The primary outcome is time alive and free of inotropes/vasopressors, censored at 7 days. Secondary outcomes include 28-day mortality, survival free of organ support, PICU length of stay, quality of life, functional status and neurodevelopmental vulnerability at 6 months post-enrollment, and hospitalization-related costs. Statistical analysis will be based on an intention-to-treat principle. The study has ethical approval (HREC/20/QCHQ/69922, dated December 21, 2020), is registered in the Australian New Zealand Clinical Trials Registry (ACTRN12621000247875), commenced recruitment on December 8, 2021, and is expected to finish recruitment by mid-2026.
Conclusions: Dissemination of the results will occur through publication in peer-reviewed journals, presentations at international conferences, and additional consumer-informed pathways.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies.
Conflict of interest statement
Drs. Gibbons and Venkatesh receive support from National Healthand Medical Research Council Investigator Grants. Dr. Venkatesh received institutional research support from Baxter. Dr. Schlapbach is supported by the Thomas & Doris Ammann Foundation and the NOMIS Foundation (Switzerland). The vitamin C used in Australian and New Zealand is provided free of cost by Biological Therapies, Australia. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
-
- Schlapbach LJ, Kissoon N: Defining pediatric sepsis. JAMA Pediatr. 2018; 172:312–314 - PubMed
-
- Weiss SL, Peters MJ, Alhazzani W, et al. : Executive summary: Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020; 21:186–195 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical