Decreased Hsp90 activity protects against TDP-43 neurotoxicity in a C. elegans model of amyotrophic lateral sclerosis
- PMID: 39724103
- PMCID: PMC11709271
- DOI: 10.1371/journal.pgen.1011518
Decreased Hsp90 activity protects against TDP-43 neurotoxicity in a C. elegans model of amyotrophic lateral sclerosis
Abstract
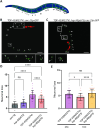
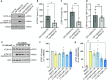
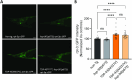
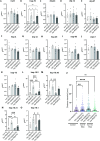
Neuronal inclusions of hyperphosphorylated TDP-43 are hallmarks of disease for most patients with amyotrophic lateral sclerosis (ALS). Mutations in TARDBP, the gene coding for TDP-43, can cause some cases of familial inherited ALS (fALS), indicating dysfunction of TDP-43 drives disease. Aggregated, phosphorylated TDP-43 may contribute to disease phenotypes; alternatively, TDP-43 aggregation may be a protective cellular response sequestering toxic protein away from the rest of the cell. The heat shock responsive chaperone Hsp90 has been shown to interact with TDP-43 and stabilize its normal conformation; however, it is not known whether this interaction contributes to neurotoxicity in vivo. Using a C. elegans model of fALS mutant TDP-43 proteinopathy, we find that loss of function of HSP-90 protects against TDP-43 neurotoxicity and subsequent neurodegeneration in adult animals. This protection is accompanied by a decrease in both total and phosphorylated TDP-43 protein. We also find that hsp-90 mutation or inhibition upregulates key stress responsive heat shock pathway gene expression, including hsp-70 and hsp-16.1, and we demonstrate that normal levels of hsp-16.1 are required for hsp-90 mutation effects on TDP-43. We also observe that the neuroprotective effect due to HSP-90 dysfunction does not involve direct regulation of proteasome activity in C. elegans. Our data demonstrate for the first time that Hsp90 chaperone activity contributes to adverse outcomes in TDP-43 proteinopathies in vivo using a whole animal model of ALS.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

