Development of a diagnostic multivariable prediction model of a positive SARS-CoV-2 RT-PCR result in healthcare workers with suspected SARS-CoV-2 infection in hospital settings
- PMID: 39724211
- PMCID: PMC11670996
- DOI: 10.1371/journal.pone.0316207
Development of a diagnostic multivariable prediction model of a positive SARS-CoV-2 RT-PCR result in healthcare workers with suspected SARS-CoV-2 infection in hospital settings
Abstract
Background: Despite declining COVID-19 incidence, healthcare workers (HCWs) still face an elevated risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection. We developed a diagnostic multivariate model to predict positive reverse transcription polymerase chain reaction (RT-PCR) results in HCWs with suspected SARS-CoV-2 infection.
Methods: We conducted a cross-sectional study on episodes involving suspected SARS-CoV-2 symptoms or close contact among HCWs in Bogotá, Colombia. Potential predictors were chosen based on clinical relevance, expert knowledge, and literature review. Logistic regression was used, and the best model was selected by evaluating model fit with Akaike Information Criterion (AIC), deviance, and maximum likelihood.
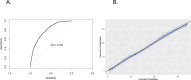
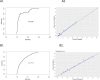
Results: The study included 2498 episodes occurring between March 6, 2020, to February 2, 2022. The selected variables were age, socioeconomic status, occupation, service, symptoms (fever, cough, fatigue/weakness, diarrhea, anosmia or dysgeusia), asthma, history of SARS-CoV-2, vaccination status, and population-level RT-PCR positivity. The model achieved an AUC of 0.79 (95% CI 0.77-0.81), with 93% specificity, 36% sensitivity, and satisfactory calibration.
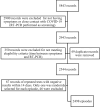
Conclusions: We present an innovative diagnostic prediction model that as a special feature includes a variable that represents SARS-CoV-2 epidemiological situation. Given its performance, we suggest using the model differently based on the level of viral circulation in the population. In low SARS-CoV-2 circulation periods, the model could serve as a replacement diagnostic test to classify HCWs as infected or not, potentially reducing the need for RT-PCR. Conversely, in high viral circulation periods, the model could be used as a triage test due to its high specificity. If the model predicts a high probability of a positive RT-PCR result, the HCW may be considered infected, and no further testing is performed. If the model indicates a low probability, the HCW should undergo a COVID-19 test. In resource-limited settings, this model can help prioritize testing and reduce expenses.
Copyright: © 2024 Valderrama-Beltrán et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Any conflicts of interest have been disclosed independently by each author in accordance with the journal’s requirements. Specifically, the authors Sandra Liliana Valderrama-Beltrán, Carlos Álvarez-Moreno, Beatriz Ariza, and Juan Sebastián Montealegre-Diaz have declared competing interests, which are not directly related to this manuscript, as detailed in the submission. This study was conducted without external funding, relying solely on resources from the Hospital Universitario San Ignacio. We confirm that these declarations do not alter our adherence to PLOS ONE policies on sharing data and materials, as outlined in the author guidelines.
Figures
References
-
- World Health Organization. Infection prevention and control in the context of COVID-19: a guideline [Internet]. 2023. [cited 2024 Apr 7]. Available from: WHO/2019-nCoV/IPC/guideline/ 2023.4.
-
- Center for Disease Control and Prevention (CDC). Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic [Internet]. 2023 May. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommen....
-
- World Health Organization 2023. WHO roadmap on uses of COVID-19 vaccines in the context of Omicron and high population immunity [Internet]. 2023 Nov [cited 2024 Apr 9]. Available from: WHO/2019-nCoV/Vaccines/SAGE/Prioritization/2023.2.
-
- Ministerio de Salud y Protección Social. Lineamientos para el uso de pruebas diagnósticas para SARS-CoV-2 (COVID-19) en Colombia. Bogotá: Ministerio de Salud y Protección Social; 2022. Available from: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP....
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

