Deciphering long-term immune effects of HIV-1/SARS-CoV-2 co-infection: a longitudinal study
- PMID: 39724280
- PMCID: PMC11671559
- DOI: 10.1007/s00430-024-00813-z
Deciphering long-term immune effects of HIV-1/SARS-CoV-2 co-infection: a longitudinal study
Erratum in
-
Correction: Deciphering long-term immune effects of HIV-1/SARS-CoV-2 co-infection: a longitudinal study.Med Microbiol Immunol. 2025 May 6;214(1):20. doi: 10.1007/s00430-025-00829-z. Med Microbiol Immunol. 2025. PMID: 40327102 Free PMC article. No abstract available.
Abstract
Introduction: While the general immune response to Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) is well-understood, the long-term effects of Human Immunodeficiency Virus-1/Severe Acute Respiratory Syndrome-Coronavirus-2 (HIV-1/SARS-CoV-2) co-infection on the immune system remain unclear. This study investigates the immune response in people with HIV-1 (PWH) co-infected with SARS-CoV-2 to understand its long-term health consequences.
Methods: A retrospective longitudinal study of PWH with suppressed viral load and SARS-CoV-2 infection was conducted. Cryopreserved peripheral blood mononuclear cells and plasma samples were collected at three time-points: HIV-1/pre-SARS-CoV-2 (n = 18), HIV-1/SARS-CoV-2 (n = 46), and HIV-1/post-SARS-CoV-2 (n = 36). Plasma levels of 25 soluble cytokines and chemokines, and anti-S/anti-N-IgG-SARS-CoV-2 antibodies were measured. Immunophenotyping of innate and adaptive immune components and HIV-1 and SARS-CoV-2-specific T/B-cell responses were assessed by flow cytometry.
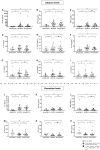
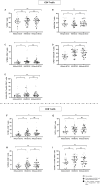
Results: HIV-1/SARS-CoV-2 co-infection was associated with long-lasting immune dysfunction, characterized by elevated levels of pro-inflammatory cytokines and a decrease in the MIG-IP10-ITAC chemokine axis at the HIV/SARS-CoV-2 time-point, which persisted one year later. Additionally, alterations in the distribution of subsets and increased activation (NKG2D/NKG2C) and maturation (TIM3) markers of NK and dendritic cells were observed at the HIV-1/SARS-CoV-2 time-point, persisting throughout the study. Effector memory CD4 T-cell subsets were decreased, while exhaustion/senescence (PD1/TIM3/CD57) markers were elevated at all three time-points. SARS-CoV-2-specific T/B-cell responses remained stable throughout the study, while HIV-1-specific T-cell responses decreased at the HIV-1/SARS-CoV-2 time-point and remained so.
Conclusions: Persistent immune dysfunction in HIV-1/SARS-CoV-2 co-infection increases the risk of future complications, even in PWH with mild symptoms. Exacerbated inflammation and alterations in immune cells may contribute to reduce vaccine efficacy and potential reinfections.
Keywords: Dysregulated immune profile; HIV-1; HIV-1 immunovirological parameters; Longitudinal analysis; SARS-CoV-2; SARS-CoV-2-specific T/B-cell responses.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- CB22/01/00041/Centro de Investigación Biomédica en Red en Bioingeniería, Biomateriales y Nanomedicina
- CB22/01/00041/Centro de Investigación Biomédica en Red en Bioingeniería, Biomateriales y Nanomedicina
- CP23/00009/Instituto de Salud Carlos III
- PI19/01638/Instituto de Salud Carlos III
- PT23/00148/Strategic Health Action of the State Plan for Scientific, Technical and Innovation Research 2021-2023
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

