Efficacy of a 2-MNG-Containing Depigmenting Serum in the Treatment of Post-Inflammatory Hyperpigmentation
- PMID: 39724317
- PMCID: PMC11837230
- DOI: 10.1111/jocd.16735
Efficacy of a 2-MNG-Containing Depigmenting Serum in the Treatment of Post-Inflammatory Hyperpigmentation
Abstract
Background: Post-inflammatory hyperpigmentation (PIHP) predominantly affects patients with melanin-rich skin, significantly impacting them psychosocially due to more frequent and severe pigmentary changes. In this study, the efficacy of a novel depigmenting agent 2-mercaptonicotinoyl glycine (Melasyl) in a dermocosmetic (DC) serum formulation is assessed as a stand-alone treatment of PIHP without sunscreen.
Materials and methods: Thirty-two Mauritian subjects aged 18-50 years of phototype IV-VI presenting mild acne (GEA2) and moderate to severe PIHP (PAHPI > 10) participated in this study. Subjects applied the DC serum twice a day on the whole face for 3 months. Efficacy was assessed through PAHPI score, mean darkness of lesions (0-8 scores), and colorimetric measurements at D0, D14, D28, D56, and D84. Self-perceived efficacy, tolerability, stigmatization, cosmeticity, and satisfaction were also gathered.
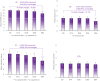
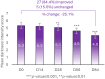
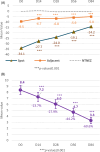
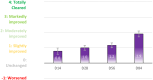
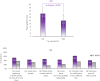
Results: A significant decrease of 15.8% (p < 0.05) in PAHPI score was reported at D84. The PAHPI score showed marked changes (p < 0.05) in pigmentation intensity at D56. Visible significant changes in mean darkness were observed from D28, with a 25.1% decrease at D84. A significant brightening effect was observed in both the spots and adjacent areas, with their mean ITA values increasingly converging toward the ITA of the non-treated, non-exposed zone. This effect was more pronounced in the spots, which showed a significant increase from -34.1 to -14.2 by D14. Instrumental measurements revealed a 60% reduction in spot color intensity at D84 compared to the adjacent area Delta E (p < 0.05), significant from D14. Subjects reported self-perceived improvements in appearance and well-being that matched clinical results, enhancing their quality of life and satisfaction. The product was reported as very well tolerated by the subjects after 84 days of usage.
Conclusion: This study demonstrated the efficacy and tolerability of the serum in the treatment of post acne PIHP. Good clinical results are confirmed by the objective measurement of the ITA and Delta E. Cosmeticity was excellent and will help observance in real life. The reduction of the stigmatization score illustrates the impact of PIHP and its improvement over time. These results can be considered highly encouraging with the new serum, as in real life, its use in combination with a UVA/visible light filtration sunscreen would probably increase patients benefit.
Keywords: PIHP; depigmenting; efficacy; hyperpigmentation; skincare.
© 2024 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
A.L.D.‐F., G.L.D., D.K., and C.L.F. are all employees of L'Oréal. G.P. and D.J. are employees of CIDP Ltée, the CRO that conducted the study.
Figures

References
-
- Gimenez Garcia R. M. and Carrasco Molina S., “Drug‐Induced Hyperpigmentation: Review and Case Series,” Journal of American Board of Family Medicine 32, no. 4 (2019): 628–638. - PubMed
-
- Sutaria A. H., Masood S., Saleh H. M., and Schlessinger J., Acne Vulgaris (Treasure Island, FL: StatPearls Publishing LLC, 2023). - PubMed
-
- Abad‐Casintahan F., Chow S. K., Goh C. L., et al., “Frequency and Characteristics of Acne‐Related Post‐Inflammatory Hyperpigmentation,” Journal of Dermatology 43, no. 7 (2016): 826–828. - PubMed
-
- Maghfour J., Olayinka J., Hamzavi I. H., and Mohammad T. F., “A Focused Review on the Pathophysiology of Post‐Inflammatory Hyperpigmentation,” Pigment Cell & Melanoma Research 35, no. 3 (2022): 320–327. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

