Long-Term Safety and Effectiveness of Canakinumab in Patients with MKD/HIDS: Interim Analysis of the RELIANCE Registry
- PMID: 39724475
- PMCID: PMC11751260
- DOI: 10.1007/s40744-024-00733-7
Long-Term Safety and Effectiveness of Canakinumab in Patients with MKD/HIDS: Interim Analysis of the RELIANCE Registry
Abstract
Introduction: Interim analysis of the long-term safety and effectiveness of canakinumab, at a patient level, in the mevalonate kinase deficiency/hyperimmunoglobulin-D syndrome (MKD/HIDS) cohort of the RELIANCE registry.
Methods: From June 2018, the RELIANCE registry enrolled paediatric (aged ≥ 2 years) and adult patients (aged ≥ 18 years) with MKD/HIDS who were receiving canakinumab as part of their routine medical care. Safety, physician- and patient-reported measures of disease activity and dosing patterns were evaluated at baseline and every 6 months until end-of-study visit.
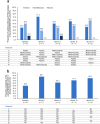
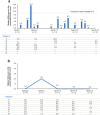
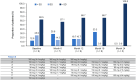
Results: At the analysis cut-off date of December 2022, eight patients with MKD/HIDS were enrolled. Five (62.5%) were children (< 18 years) and five (62.5%) were female. The median patient age was 8.0 (range 2.0-39.0) years, and all patients were pre-treated with canakinumab prior to enrolment (median duration of canakinumab treatment: 3.8 years). Canakinumab was well tolerated, with seven (87.5%) patients reporting 48 adverse events (incidence rate/100 patient years: 218.1). No serious adverse drug reactions were reported. Patients continued to receive vaccinations during long-term treatment with canakinumab. Disease activity, evaluated by physician-reported (physician's global assessment, disease remission, C-reactive protein, serum amyloid A, erythrocyte sedimentation rate) and patient-reported (autoinflammatory disease activity index diary, disease activity, fatigue, impact on social life) measures, was generally well controlled throughout the study. Over 50.0% of patients maintained disease remission from baseline to month 24, and medians of all inflammatory markers remained within normal limits throughout the study. Most patients received higher than the recommended starting dose of canakinumab throughout the study.
Conclusion: Data from this interim analysis of a unique registry of patients with a rare disease support the long-term safety and effectiveness of the IL-1-blocking agent canakinumab for the treatment of MKD/HIDS.
Keywords: Autoinflammatory disease; Canakinumab; Fever syndromes; Hyperimmunoglobulin-d syndrome; Mevalonate kinase deficiency.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Prasad T. Oommen has received study support from Novartis. Tilmann Kallinich received research support from Novartis. Juergen Rech has received grants from Novartis and Sobi; speaker fees from BMS, Novartis and Sobi; support for attending meetings and travel from BMS; and has participated on a data safety monitoring board and/or advisory board for BMS, Novartis, Sobi. Norbert Blank has received grant/research support from Novartis and Sobi; is a consultant of Novartis, Sobi, Lilly, Pfizer, AbbVie, BMS, MSD, Actelion, UCB, Boehringer Ingelheim and Roche. Julia Weber-Arden was an employee of Novartis at the time of data acquisition and drafting of the manuscript and now is an employee of Fresenius Kabi at the time of publication. Jasmin B. Kuemmerle-Deschner has received grant/research support from Novartis and Sobi and is a consultant of Novartis and Sobi. Ethical Approval: The study was conducted according to the principles of the Declaration of Helsinki. The Ethics Committee of the Medical Faculty and the University Hospital Tübingen, as the leading ethics committee, and the local ethics committees of participating institutions, i.e., The Ethics Committee of Charité-Universitätsmedizin Berlin, The Ethics Committee of the Friedrich-Alexander University Erlangen-Nuremberg, The Heidelberg Ethics Committee and The Ethics Committee at the Faculty of Medicine of Heinrich Heine University Düsseldorf, Germany, approved the protocol used in this registry. Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of the research.
Figures



Similar articles
-
Long-term safety and effectiveness of canakinumab in patients with monogenic autoinflammatory diseases: results from the interim analysis of the RELIANCE registry.RMD Open. 2024 Feb 15;10(1):e003890. doi: 10.1136/rmdopen-2023-003890. RMD Open. 2024. PMID: 38360038 Free PMC article.
-
Canakinumab treatment patterns in sJIA, FMF, TRAPS, and MKD/HIDS: real-world insights from a Belgian non-interventional study.BMC Rheumatol. 2025 May 29;9(1):64. doi: 10.1186/s41927-025-00515-w. BMC Rheumatol. 2025. PMID: 40442768 Free PMC article.
-
Real-world safety and effectiveness of canakinumab in patients with tumour necrosis factor receptor-associated periodic syndrome or hyperimmunoglobulinaemia D syndrome: Interim results from post-marketing surveillance in Japan.Mod Rheumatol. 2023 Mar 2;33(2):381-391. doi: 10.1093/mr/roac041. Mod Rheumatol. 2023. PMID: 35575279
-
Systematic literature review of efficacy/effectiveness and safety of current therapies for the treatment of cryopyrin-associated periodic syndrome, hyperimmunoglobulin D syndrome and tumour necrosis factor receptor-associated periodic syndrome.RMD Open. 2020 Jul;6(2):e001227. doi: 10.1136/rmdopen-2020-001227. RMD Open. 2020. PMID: 32723831 Free PMC article.
-
Consensus protocols for the diagnosis and management of the hereditary autoinflammatory syndromes CAPS, TRAPS and MKD/HIDS: a German PRO-KIND initiative.Pediatr Rheumatol Online J. 2020 Feb 17;18(1):17. doi: 10.1186/s12969-020-0409-3. Pediatr Rheumatol Online J. 2020. PMID: 32066461 Free PMC article.
References
-
- Jeyaratnam J, Simon A, Calvo I, Constantin T, Shcherbina A, Hofer M, et al. Long-term efficacy and safety of canakinumab in patients with mevalonate kinase deficiency: results from the randomised phase 3 CLUSTER trial. Rheumatology (Oxford). 2022;61:2088–94. - PubMed
-
- Brennenstuhl H, Nashawi M, Schröter J, Baronio F, Beedgen L, Gleich F, et al. Phenotypic diversity, disease progression, and pathogenicity of MVK missense variants in mevalonic aciduria. J Inherit Metab Dis. 2021;44:1272–87. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

