Efficacy and Safety of Nefecon in Patients with IgA Nephropathy from Mainland China: 2-Year NefIgArd Trial Results
- PMID: 39724565
- PMCID: PMC11687989
- DOI: 10.34067/KID.0000000583
Efficacy and Safety of Nefecon in Patients with IgA Nephropathy from Mainland China: 2-Year NefIgArd Trial Results
Abstract
Background: IgA nephropathy (IgAN), an immune-mediated kidney disease, is particularly prevalent among individuals of East Asian ancestry. Nefecon is a novel, oral, targeted-release budesonide formulation designed to inhibit galactose-deficient IgA1 formation underlying IgAN pathophysiology. We present findings in patients with IgAN from mainland China participating in the 2-year, multicenter, randomized, double-blind, phase 3 NefIgArd trial of nefecon.
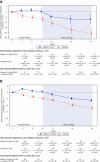
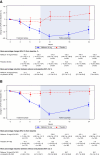
Methods: Patients (aged 18 years and older) with primary IgAN (eGFR 35–90 ml/min per 1.73 m2, persistent proteinuria [urine protein–creatinine ratio ≥0.8 g/g or proteinuria ≥1 g/24 hours] despite optimized renin-angiotensin system blockade) received nefecon or placebo over 9 months, followed by a 15-month follow-up phase on supportive care alone. The primary efficacy end point was time-weighted average of eGFR over 2 years.
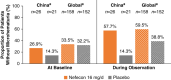
Results: Sixty-two patients from mainland China were included in this prespecified analysis. The primary efficacy end point was 9.6 ml/min per 1.73 m2 (95% confidence interval, 2.0 to 19.8) in favor of nefecon versus placebo. This was consistent with (and numerically greater than) that of the global study population. Time to confirmed 30% eGFR reduction or kidney failure from baseline was substantially delayed with nefecon (patients with an event: 9%) versus placebo (30%; hazard ratio, 0.21; 95% confidence interval, 0.04 to 0.73). No deaths were reported in the China cohort. In the nefecon group, treatment-emergent serious adverse events were reported by one patient during treatment and two patients during follow-up (versus no patients and seven patients, respectively, in the placebo group). No severe infections requiring hospitalization were reported.
Conclusions: Nefecon treatment for 9 months showed greater preservation of eGFR over 2 years compared with placebo. The efficacy outcomes were consistent with global study results, with a numerically greater treatment benefit observed in patients from China. Nefecon was well tolerated, with no unexpected safety signals.
Trial registration: ClinicalTrials.gov NCT03643965.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
Disclosure forms, as provided by each author, are available with the online version of the article at
Figures


References
-
- Lafayette R Kristensen J Stone A, et al.; NefIgArd trial investigators. Efficacy and safety of a targeted-release formulation of budesonide in patients with primary IgA nephropathy (NefIgArd): 2-year results from a randomised phase 3 trial. Lancet. 2023;402(10405):859–870. doi: 10.1016/S0140-6736(23)01554-4 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

