[Mechanism of WAVE1 regulation of lipopolysaccharide-induced mitochondrial metabolic abnormalities and inflammatory responses in macrophages]
- PMID: 39725399
- PMCID: PMC11684833
- DOI: 10.7499/j.issn.1008-8830.2408083
[Mechanism of WAVE1 regulation of lipopolysaccharide-induced mitochondrial metabolic abnormalities and inflammatory responses in macrophages]
Abstract
Objectives: To explore the mechanism by which Wiskott-Aldrich syndrome protein family verprolin-homologous protein 1 (WAVE1) regulates lipopolysaccharide (LPS)-induced mitochondrial metabolic abnormalities and inflammatory responses in macrophages.
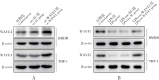
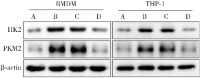
Methods: Macrophage cell lines with overexpressed WAVE1 (mouse BMDM and human THP1 cells) were prepared. The macrophages were treated with LPS (500 ng/mL) to simulate sepsis-induced inflammatory responses. The experiment consisted of two parts. The first part included control, LPS, vector (LPS+oe-NC), WAVE1 overexpression (LPS+oe-WAVE1) groups. The second part included LPS, LPS+oe-NC, LPS+oe-WAVE1 and exogenous high mobility group box-1 (HMGB1) intervention (LPS+oe-WAVE1+HMGB1) groups. RT-PCR was used to measure mitochondrial DNA content, and RT-qPCR was used to detect the mRNA expression levels of WAVE1, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6. Western blot was performed to measure the protein expression of WAVE1, hexokinase 2, and pyruvate kinase M2. ELISA was utilized to detect the levels of TNF-α, IL-1β, IL-6, and HMGB1. JC-1 staining was used to assess mitochondrial membrane potential. Seahorse XP96 was used to evaluate oxygen consumption rate and extracellular acidification rate. MitoSOX probe was employed to measure mitochondrial reactive oxygen species levels, and 2-NBDG method was used to assess glucose uptake. Kits were used to measure pyruvate kinase activity, lactate, adenosine triphosphate (ATP), and HMGB1 levels.
Results: Compared with the control group, the LPS group showed lower levels of WAVE1 protein and mRNA expression, mitochondrial membrane potential, oxygen consumption rate, and mitochondrial DNA content (P<0.05), while TNF-α, IL-1β, IL-6 levels and mRNA expression, mitochondrial reactive oxygen species, glucose uptake, lactate, ATP, hexokinase 2, and pyruvate kinase M2 protein expression levels as well as extracellular acidification rate, pyruvate kinase activity, and HMGB1 release were significantly increased (P<0.05). Compared with the LPS+oe-NC group, the LPS+oe-WAVE1 group showed increased WAVE1 protein and mRNA expression, mitochondrial membrane potential, oxygen consumption rate, and mitochondrial DNA content (P<0.05), while TNF-α, IL-1β, IL-6 levels and mRNA expression, mitochondrial reactive oxygen species, glucose uptake, lactate, ATP, hexokinase 2, and pyruvate kinase M2 protein expressions, as well as extracellular acidification rate, pyruvate kinase activity, and HMGB1 release were decreased (P<0.05). Compared with the LPS+oe-WAVE1 group, the LPS+oe-WAVE1+HMGB1 group exhibited increased glucose uptake, lactate, ATP levels, and extracellular acidification rate (P<0.05).
Conclusions: WAVE1 participates in the regulation of LPS-induced inflammatory responses in macrophages by modulating the release of inflammatory factors, mitochondrial metabolism, and HMGB1 release.
目的: 探讨WASP家族富含脯氨酸同源蛋白1(Wiskott-Aldrich syndrome protein family verprolin-homologous protein 1, WAVE1)调控脂多糖(lipopolysaccharide, LPS)诱导的巨噬细胞线粒体代谢异常和炎症反应的机制。方法: 构建过表达WAVE1的巨噬细胞系(小鼠BMDM和人THP-1细胞),LPS(500 ng/mL)处理巨噬细胞模拟脓毒症炎症反应,实验分为两部分。第一部分设立对照组、LPS组、空载质粒(LPS+oe-NC)组、WAVE1过表达(LPS+oe-WAVE1)组;第二部分设立LPS组、LPS+oe-NC组、LPS+oe-WAVE1组、外源性高迁移率族蛋白1(high mobility group box-1, HMGB1)干预(LPS+oe-WAVE1+HMGB1)组。采用RT-PCR法测定线粒体DNA含量,RT-qPCR法检测WAVE1、肿瘤坏死因子-α(tumor necrosis factor-α, TNF-α)、白介素(interleukin, IL)-1β、IL-6 mRNA表达,免疫印迹法检测WAVE1、己糖激酶2、丙酮酸激酶M2蛋白表达,ELISA法检测TNF-α、IL-1β、IL-6、HMGB1含量,JC-1染色法检测线粒体膜电位,Seahorse XP96法检测耗氧率和细胞外酸化率,MitoSOX探针检测线粒体活性氧水平,2-NBDG法检测葡萄糖摄取水平,试剂盒检测丙酮酸激酶活性、乳酸、三磷酸腺苷、HMGB1水平。结果: 与对照组相比,LPS组WAVE1蛋白和mRNA表达、线粒体膜电位、耗氧率、线粒体DNA含量降低(P<0.05),TNF-α、IL-1β、IL-6含量和mRNA表达、线粒体活性氧、葡萄糖摄取、乳酸、三磷酸腺苷、己糖激酶2和丙酮酸激酶M2蛋白表达水平以及细胞外酸化率、丙酮酸激酶活性、HMGB1释放量升高(P<0.05);与LPS+oe-NC组相比,LPS+oe-WAVE1组WAVE1蛋白和mRNA表达、线粒体膜电位、耗氧率、线粒体DNA含量升高(P<0.05),TNF-α、IL-1β、IL-6含量和mRNA表达、线粒体活性氧、葡萄糖摄取、乳酸、三磷酸腺苷、己糖激酶2和丙酮酸激酶M2蛋白表达水平以及细胞外酸化率、丙酮酸激酶活性、HMGB1释放量降低(P<0.05)。与LPS+oe-WAVE1组比较,LPS+oe-WAVE1+HMGB1组葡萄糖摄取、乳酸、三磷酸腺苷水平及细胞外酸化率升高(P<0.05)。结论: WAVE1通过调控炎症因子释放、线粒体代谢及HMGB1释放参与调控LPS诱导的巨噬细胞炎症反应。.
Keywords: High mobility group box-1; Macrophage; Mitochondrial metabolism; Sepsis; WAVE1; Warburg effect.
Conflict of interest statement
所有作者均声明不存在利益冲突。
Figures



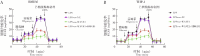
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

