Has the UK lost its position as a destination for world-leading clinical research? A comparative analysis of haematological cancer clinical trials performance before Brexit
- PMID: 39725437
- PMCID: PMC11683988
- DOI: 10.1136/bmjopen-2024-086058
Has the UK lost its position as a destination for world-leading clinical research? A comparative analysis of haematological cancer clinical trials performance before Brexit
Abstract
Objectives: To understand the competitive position of the UK in comparison to Europe and the USA for haematological cancer clinical research.
Design: Using commercially available databases, clinical trial numbers, their effectiveness and publication outputs were evaluated in two analyses: a macrodevelopment and a research activity and performance analysis.
Data sources: The following databases were used for this analysis: Organisation for Economic Co-operation and Development, Thomson Reuters Incidence and Prevalence, the Cortellis Clinical Trial Intelligence, the Clarivate Cortellis Innography Patent Intelligence, Thomson-Reuters Cortellis Regulatory Intelligence, Thomson Reuters Web of Science and data from the Centre for Medicine Research (CMR).
Eligibility criteria: European countries with comparable geography, healthcare standards and economies, as well as the USA, the largest country where research is conducted. All haematological oncology clinical trials from phase 1 to phase 3 were included.
Data extraction and synthesis: All data were retrieved in September 2017 and macroeconomic data were reviewed in 2022; haematological cancer data were restricted to leukaemias generally as a surrogate reference for haematological oncology indications; research output publication data were evaluated using specific MeSH/keyword search terms between 2010 and 2017. Key metrics explored included healthcare expenditure per capita, study experience across countries, comparative capability of each country for clinical trial implementation, clinical trials' performance and impact of research as measured by impact factor and citation metrics of publications.
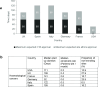
Results: Revenue for clinical studies is lower in the UK than European comparators. All studied countries had comparable leukaemia prevalence rates, but the UK spent least per capita on healthcare versus France, Germany, Spain and the USA. The number of clinical studies in the UK showed a decline compared with other European countries. Clinical trial implementation was lowest in the UK (n=380) versus Germany (n=665), France (n=643), Spain (n=632), Italy (n=538) and the USA (n=3254). Registered versus active clinical studies suggested the USA had the highest number underway (n=824), with the UK ranked fourth of five European countries (Germany=239, Italy=232, France=211, UK=177 and Spain=141). However, the UK had the highest completion rate of phase 3 studies it did initiate (n=154, 87%) and performance was comparable with Germany (n=188, 78.7%) and France (n=151, 71.6%). When analysed by phase, the UK was the second highest European performing country (n=121) for phase 2 study completion compared with Germany (n=182) both less than the USA (n=345). The UK completed the most phase 1 studies compared with other European countries, only second to the USA (n=31 vs n=126). However, the UK clinical trial performance metrics were negatively impacted for the UK compared with other European countries with respect to clinical trial application (CTA) process, timelines, ethics committee approvals, median time to start up and rate of non-enrolling sites. The UK was slower to initiate studies (median 186 days) vs Germany (92 days), France (141 days), Italy (122 days) and only marginally faster than Spain (195 days). While median enrolment rates were comparable across all countries, the UK had the highest proportion of sites that failed to enrol any patients (despite regulatory timings being comparable to Germany (90 days) and France (95 days)). However, publication of data following clinical trials in the UK was robust and of the highest quality compared with other countries, judged by journal placement and publication citations. The UK published high-quality, diverse research with citation rates (11.8) from clinical studies which was higher than every other country, including the USA who published fivefold more publications per year.
Conclusion: While research in the UK remains among the highest quality and value globally, the UK may be losing its position globally as an attractive destination for executing clinical trials. This may be a trend that is recognised by the UK Government, but it is vital to reverse the trend of clinical trial decline and to improve the economic outlook for the UK and patient early access to innovative cancer medicines.
Keywords: Clinical Decision-Making; Health Equity; Leukaemia.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The authors declare there are no current conflicts of interest. MA, JB and AK were employees of Celgene at the time the analysis was conducted. The analysis was conducted as part of research for a successfully defended MSc thesis for MA, which was funded by Celgene and Amryt Pharmaceuticals.
Figures


Similar articles
-
Trends of Phase I Clinical Trials in the Latest Ten Years across Five European Countries.Int J Environ Res Public Health. 2022 Oct 28;19(21):14023. doi: 10.3390/ijerph192114023. Int J Environ Res Public Health. 2022. PMID: 36360902 Free PMC article.
-
Efaproxiral: GSJ 61, JP 4, KDD 86, RS 4, RSR 13.Drugs R D. 2005;6(3):178-85. doi: 10.2165/00126839-200506030-00007. Drugs R D. 2005. PMID: 15869322 Review.
-
Impact assessment of the European Clinical Trials Directive: a longitudinal, prospective, observational study analyzing patterns and trends in clinical drug trial applications submitted since 2001 to regulatory agencies in six EU countries.Trials. 2012 Apr 29;13:53. doi: 10.1186/1745-6215-13-53. Trials. 2012. PMID: 22540886 Free PMC article.
-
Orthopedics research output from China, USA, UK, Japan, Germany and France: A 10-year survey of the literature.Orthop Traumatol Surg Res. 2016 Nov;102(7):939-945. doi: 10.1016/j.otsr.2016.05.005. Epub 2016 Jun 11. Orthop Traumatol Surg Res. 2016. PMID: 27296711
-
Variability of cost-effectiveness estimates for pharmaceuticals in Western Europe: lessons for inferring generalizability.Value Health. 2005 Jan-Feb;8(1):10-23. doi: 10.1111/j.1524-4733.2005.03070.x. Value Health. 2005. PMID: 15841890 Review.
References
-
- FADOI The value of independent clinical research in Italy. Edra Roche Foundation. 2019. https://www.fondazioneroche.it/ Available.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
