Functional Regrowth of Norepinephrine Axons in the Adult Mouse Brain Following Injury
- PMID: 39725517
- PMCID: PMC11729145
- DOI: 10.1523/ENEURO.0418-24.2024
Functional Regrowth of Norepinephrine Axons in the Adult Mouse Brain Following Injury
Abstract
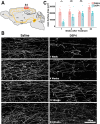
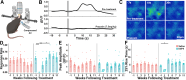
It is widely believed that axons in the central nervous system of adult mammals do not regrow following injury. This failure is thought, at least in part, to underlie the limited recovery of function following injury to the brain or spinal cord. Some studies of fixed tissue have suggested that, counter to dogma, norepinephrine (NE) axons regrow following brain injury. Here, we have used in vivo two-photon microscopy in layer 1 of the primary somatosensory cortex in transgenic mice harboring a fluorophore selectively expressed in NE neurons. This protocol allowed us to explore the dynamic nature of NE axons following injury with the selective NE axon toxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP4). Following DSP4, NE axons were massively depleted and then slowly and partially recovered their density over a period of weeks. This regrowth was dominated by new axons entering the imaged volume. There was almost no contribution from local sprouting from spared NE axons. Regrown axons did not appear to use either the paths of previously lesioned NE axons or NE axons that were spared and survived DSP4 as a guide. To measure NE release, GCaMP8s was selectively expressed in neocortical astrocytes and startle-evoked, NE receptor-mediated Ca2+ transients were measured. These Ca2+ transients were abolished soon after DSP4 lesion but returned to pre-lesion values after 3-5 weeks, roughly coincident with NE axon regrowth, suggesting that the regrown NE axons are competent to release NE in response to a physiological stimulus in the awake mouse.
Keywords: axon regeneration; in vivo microscopy; norepinephrine.
Copyright © 2025 Cooke and Linden.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Regrowth of Serotonin Axons in the Adult Mouse Brain Following Injury.Neuron. 2016 Aug 17;91(4):748-762. doi: 10.1016/j.neuron.2016.07.024. Epub 2016 Aug 4. Neuron. 2016. PMID: 27499084 Free PMC article.
-
The neurotoxic compound N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP4) depletes endogenous norepinephrine and enhances release of [3H]norepinephrine from rat cortical slices.J Pharmacol Exp Ther. 1984 Oct;231(1):131-6. J Pharmacol Exp Ther. 1984. PMID: 6491970
-
Catecholaminergic axons in the neocortex of adult mice regrow following brain injury.Exp Neurol. 2020 Jan;323:113089. doi: 10.1016/j.expneurol.2019.113089. Epub 2019 Nov 4. Exp Neurol. 2020. PMID: 31697941 Free PMC article.
-
Signaling pathways that regulate axon regeneration.Neurosci Bull. 2013 Aug;29(4):411-20. doi: 10.1007/s12264-013-1357-4. Epub 2013 Jul 11. Neurosci Bull. 2013. PMID: 23846598 Free PMC article. Review.
-
The pros and cons of growth factors and cytokines in peripheral axon regeneration.Int Rev Neurobiol. 2013;108:137-71. doi: 10.1016/B978-0-12-410499-0.00006-X. Int Rev Neurobiol. 2013. PMID: 24083434 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
