Targeting Tiam1 Enhances Hippocampal-Dependent Learning and Memory in the Adult Brain and Promotes NMDA Receptor-Mediated Synaptic Plasticity and Function
- PMID: 39725519
- PMCID: PMC11800756
- DOI: 10.1523/JNEUROSCI.0298-24.2024
Targeting Tiam1 Enhances Hippocampal-Dependent Learning and Memory in the Adult Brain and Promotes NMDA Receptor-Mediated Synaptic Plasticity and Function
Abstract
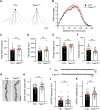
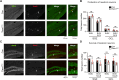
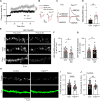
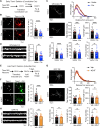
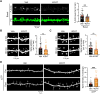
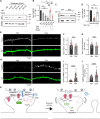
Excitatory synapses and the actin-rich dendritic spines on which they reside are indispensable for information processing and storage in the brain. In the adult hippocampus, excitatory synapses must balance plasticity and stability to support learning and memory. However, the mechanisms governing this balance remain poorly understood. Tiam1 is an actin cytoskeleton regulator prominently expressed in the dentate gyrus (DG) throughout life. Previously, we showed that Tiam1 promotes dentate granule cell synapse and spine stabilization during development, but its role in the adult hippocampus remains unclear. Here, we deleted Tiam1 from adult forebrain excitatory neurons (Tiam1fKO ) and assessed the effects on hippocampal-dependent behaviors. Adult male and female Tiam1fKO mice displayed enhanced contextual fear memory, fear extinction, and spatial discrimination. Investigation into underlying mechanisms revealed that dentate granule cells from Tiam1fKO brain slices exhibited augmented synaptic plasticity and N-methyl-D-aspartate-type glutamate receptor (NMDAR) function. Additionally, Tiam1 loss in primary hippocampal neurons blocked agonist-induced NMDAR internalization, reduced filamentous actin levels, and promoted activity-dependent spine remodeling. Notably, strong NMDAR activation in wild-type hippocampal neurons triggered Tiam1 loss from spines. Our results suggest that Tiam1 normally constrains hippocampal-dependent learning and memory in the adult brain by restricting NMDAR-mediated synaptic plasticity in the DG. We propose that Tiam1 achieves this by limiting NMDAR availability at synaptic membranes and stabilizing spine actin cytoskeleton and that these constraints can be alleviated by activity-dependent degradation of Tiam1. These findings reveal a previously unknown mechanism restricting hippocampal synaptic plasticity and highlight Tiam1 as a therapeutic target for enhancing cognitive function.
Keywords: NMDAR; Tiam1; actin cytoskeleton; dendritic spines; hippocampus; learning and memory.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
The Rac-GEF Tiam1 Promotes Dendrite and Synapse Stabilization of Dentate Granule Cells and Restricts Hippocampal-Dependent Memory Functions.J Neurosci. 2021 Feb 10;41(6):1191-1206. doi: 10.1523/JNEUROSCI.3271-17.2020. Epub 2020 Dec 16. J Neurosci. 2021. PMID: 33328293 Free PMC article.
-
Individual NMDA receptor GluN2 subunit signaling domains differentially regulate the postnatal maturation of hippocampal excitatory synaptic transmission and plasticity but not dendritic morphology.Synapse. 2024 Jul;78(4):e22292. doi: 10.1002/syn.22292. Synapse. 2024. PMID: 38813758 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Memory processing by hippocampal adult-born neurons.Neurobiol Learn Mem. 2025 Jul;220:108062. doi: 10.1016/j.nlm.2025.108062. Epub 2025 May 8. Neurobiol Learn Mem. 2025. PMID: 40345378 Review.
-
Short term plasticity at hippocampal mossy fiber synapses.Neuroscience. 2025 Jul 10;578:105-117. doi: 10.1016/j.neuroscience.2024.09.044. Epub 2024 Sep 26. Neuroscience. 2025. PMID: 39332701 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
