[Parkin deletion affects PINK1/Parkin-mediated mitochondrial autophagy to exacerbate neuroinflammation and accelerate progression of Parkinson's disease in mice]
- PMID: 39725624
- PMCID: PMC11683357
- DOI: 10.12122/j.issn.1673-4254.2024.12.11
[Parkin deletion affects PINK1/Parkin-mediated mitochondrial autophagy to exacerbate neuroinflammation and accelerate progression of Parkinson's disease in mice]
Abstract
Objectives: To investigate the role of mitochondrial autophagy disorder caused by deletion of E3 ubiquitin ligase Parkin in neuroinflammation in a mouse model of MPTP-induced Parkinson's disease (PD).
Methods: Wild-type (WT) male C57BL/6 mice and Parkin-/- mice were given intraperitoneal injections with MPTP or PBS for 5 consecutive days, and the changes in motor behaviors of the mice were observed using open field test. The effects of Parkin deletion on PD development and neuroinflammation were evaluated using immunofluorescence and Western blotting. The changes of the PINK 1/Parkin signaling pathway in the midbrain substantia nigra of the mice were examined to explore the molecular mechanism of Parkin-mediated regulation of mitochondrial autophagy and its effect on neuroinflammation in PD mice.
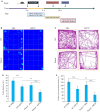
Results: Compared with their WT counterparts, the Parkin-/- mice with MPTP injections exhibited significant impairment of motor function with decreased TH+ neurons, increased α-synuclein (α-syn) accumulation, and increased numbers of GFAP+ and I-ba1+ cells in the midbrain substantia nigra. Parkin deletion obviously affected PINK1/Parkin-mediated mitochondrial autophagy to result in significantly increased mtDNA and upregulated expressions of STING and NLRP3 inflammatosomes in the midbrain substantia nigra of MPTP-treated transgenic mice.
Conclusions: Parkin deletion causes mitochondrial autophagy disorder to accelerate PD progression and exacerbates neuroinflammation in mice by affecting the PINK1/Parkin signaling pathway, suggesting the important role of Parkin in early pathogenesis of PD.
目的: 探讨E3 泛素连接酶Parkin缺失造成的线粒体自噬障碍在帕金森病(PD)神经炎症中的作用。方法: 建立MPTP-PD模型,野生型(WT)小鼠和Parkin-/-小鼠连续腹腔注射MPTP 5 d建立PD小鼠模型,分别设置对照组:WT-PBS组、Parkin-/--PBS组;PD建模组:WT-MPTP组、Parkin-/--MPTP组,8只/组,对照组连续5 d注射等量的PBS。建模1周后,通过旷场实验评估WT小鼠与Parkin-/-小鼠的运动行为;通过脑切片免疫荧光和Western blotting检测 Parkin缺失对PD发展以及神经炎症的影响;通过PINK 1/Parkin信号通路变化,探讨Parkin调控线粒体自噬对PD神经炎症发生的分子机制。结果: 与WT-MPTP组相比,Parkin-/--MPTP组小鼠运动功能下降(P<0.001),脑内TH+神经元减少并且α-突触核蛋白(α-syn)积累增加;神经炎症相关GFAP和I-ba1阳性细胞数量增加(P<0.001)。Parkin缺失影响PINK1/Parkin介导的线粒体自噬,导致mtDNA增多以及炎症相关蛋白STING和NLRP3炎症小体的表达上调(P<0.01)。结论: Parkin通过调节PINK1/Parkin信号通路加速了小鼠帕金森病发展及神经炎症发生,为后期研究Parkin基因隐性遗传早发性帕金森病发病机制及其治疗奠定了实验基础。.
Keywords: Parkin; Parkinson's disease; mitochondrial autophagy; neuroinflammation.
Figures



Similar articles
-
Effects of electroacupuncture on mitophagy mediated by SIRT3/PINK1/Parkin pathway in Parkinson's disease mice.Zhen Ci Yan Jiu. 2024 Mar 25;49(3):221-230. doi: 10.13702/j.1000-0607.20230654. Zhen Ci Yan Jiu. 2024. PMID: 38500318 Chinese, English.
-
Inhibition of NLRP3 inflammasome ameliorates LPS-induced neuroinflammatory injury in mice via PINK1/Parkin pathway.Neuropharmacology. 2024 Oct 1;257:110063. doi: 10.1016/j.neuropharm.2024.110063. Epub 2024 Jul 6. Neuropharmacology. 2024. PMID: 38972372
-
BL-918 activates PINK1/Parkin signaling pathway to ameliorate the progression of Parkinson's disease.J Biol Chem. 2024 Aug;300(8):107543. doi: 10.1016/j.jbc.2024.107543. Epub 2024 Jul 9. J Biol Chem. 2024. PMID: 38992440 Free PMC article.
-
PINK1/PARKIN signalling in neurodegeneration and neuroinflammation.Acta Neuropathol Commun. 2020 Nov 9;8(1):189. doi: 10.1186/s40478-020-01062-w. Acta Neuropathol Commun. 2020. PMID: 33168089 Free PMC article. Review.
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
References
-
- Jankovic J, Tan EK. Parkinson's disease: etiopathogenesis and treatment[J]. J Neurol Neurosurg Psychiatry, 2020, 91(8): 795-808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
