Therapeutic potential of microglial SMEK1 in regulating H3K9 lactylation in cerebral ischemia-reperfusion
- PMID: 39725685
- PMCID: PMC11671539
- DOI: 10.1038/s42003-024-07425-6
Therapeutic potential of microglial SMEK1 in regulating H3K9 lactylation in cerebral ischemia-reperfusion
Abstract
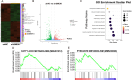
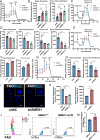
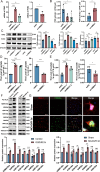
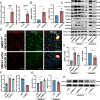
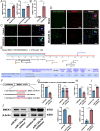
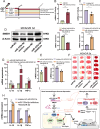
Acute ischemic stroke (AIS) triggers immune responses and neuroinflammation, contributing to brain injury. Histone lactylation, a metabolic stress-related histone modification, plays a critical role in various diseases, but its involvement in cerebral ischemia remains unclear. This study utilized a transient middle cerebral artery occlusion/reperfusion (MCAO/R) model and an oxygen-glucose deprivation/reoxygenation (OGD/R) model to investigate the role of microglial histone lactylation in ischemia-reperfusion injury. Lactate overload post-AIS increased histone lactylation, while reduced SMEK1 expression in microglia correlated with elevated lactate and neuroinflammation. Microglia-specific SMEK1 deficiency enhanced lactate production by inhibiting the pyruvate dehydrogenase kinase 3-pyruvate dehydrogenase (PDK3-PDH) pathway, increasing H3 lysine 9 lactylation (H3K9la), activating Ldha and Hif-1α transcription, and promoting glycolysis. SMEK1 overexpression improved neurological recovery in ischemic mice. This study highlights SMEK1 as a novel regulator of histone lactylation and a potential therapeutic target for mitigating neuroinflammation and enhancing recovery after AIS.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

