HFM1 is essential for the germ cell intercellular bridge transport in primordial follicle formation in mice
- PMID: 39725823
- PMCID: PMC11671464
- DOI: 10.1007/s00018-024-05541-4
HFM1 is essential for the germ cell intercellular bridge transport in primordial follicle formation in mice
Abstract
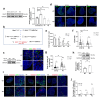
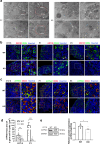
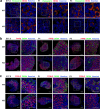
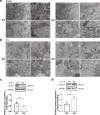
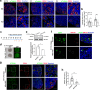
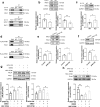
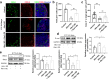
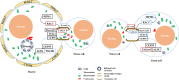
The reproductive lifespan of female mammals is determined by the size of the primordial follicle pool, which comprises oocytes enclosed by a layer of flattened pre-granulosa cells. Oocyte differentiation needs acquiring organelles and cytoplasm from sister germ cells in cysts, but the mechanisms regulating this process remain unknown. Previously helicase for meiosis 1 (HFM1) is reported to be related to the development of premature ovarian insufficiency. Here, it is found that HFM1 is involved in oocyte differentiation through organelle enrichment from sister germ cells. Further study indicates that HFM1 is involved in intercellular directional transport through intercellular bridges via the RAC1/ANLN/E-cad signaling pathway, which is indispensable for oocyte differentiation and primordial follicle formation. These findings shed light on the critical role of HFM1 in intercellular bridge transport, which is essential for the establishment of the primordial follicle pool and presenting new horizons for female fertility protection.
Keywords: HFM1; Intercellular bridge; Oocyte differentiation; POI; Primordial follicle formation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Mouse breeding conditions and breeding methods in this experiment strictly complied with the Committee on the Ethics of Animal Experiments of Nanjing Medical University. Consent to publish: All authors read and approved the submission and final publication. Conflict of interest: The authors have no relevant financial or non-financial interests to disclose.
Figures
References
-
- Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B et al (2016) ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 31:926–937. 10.1093/humrep/dew027 - PubMed
-
- Panay N, Anderson RA, Nappi RE, Vincent AJ, Vujovic S, Webber L et al (2020) Premature ovarian insufficiency: an International Menopause Society White Paper. Climacteric 23:426–446. 10.1080/13697137.2020.1804547 - PubMed
-
- Huhtaniemi I, Hovatta O, La Marca A, Livera G, Monniaux D, Persani L et al (2018) Advances in the Molecular Pathophysiology, Genetics, and Treatment of Primary Ovarian Insufficiency. Trends Endocrinol Metab 29:400–419. 10.1016/j.tem.2018.03.010 - PubMed
-
- Pu D, Wang C, Cao J, Shen Y, Jiang H, Liu J et al (2016) Association analysis between HFM1 variation and primary ovarian insufficiency in Chinese women. Clin Genet 89:597–602. 10.1111/cge.12718 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

