Measuring the biomechanical properties of cell-derived fibronectin fibrils
- PMID: 39725835
- PMCID: PMC12055646
- DOI: 10.1007/s10237-024-01918-3
Measuring the biomechanical properties of cell-derived fibronectin fibrils
Abstract
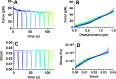
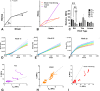
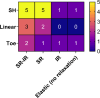
Embryonic development, wound healing, and organogenesis all require assembly of the extracellular matrix protein fibronectin (FN) into insoluble, viscoelastic fibrils. FN fibrils mediate cell migration, force generation, angiogenic sprouting, and collagen deposition. While the critical role of FN fibrils has long been appreciated, we still have an extremely poor understanding of their mechanical properties and how these mechanical properties facilitate cellular responses. Here, we demonstrate the development of a system to probe the mechanics of cell-derived FN fibrils and present quantified mechanical properties of these fibrils. We demonstrate that: fibril elasticity can be classified into three phenotypes: linearly elastic, strain-hardening, or nonlinear with a "toe" region; fibrils exhibit pre-conditioning, with nonlinear "toe" fibrils becoming more linear with repeated stretch and strain-hardened fibrils becoming less linear with repeated stretch; fibrils exhibit an average elastic modulus of roughly 8 MPa; and fibrils exhibit a time-dependent viscoelastic behavior, exhibiting a transition from a stress relaxation response to an inverse stress relaxation response. These findings have a potentially significant impact on our understanding of cellular mechanical responses in fibrotic diseases and embryonic development, where FN fibrils play a major role.
Keywords: Biomechanics; Elastic modulus; Extracellular matrix; Fibrils; Fibronectin; Mechanobiology; Nonlinear elasticity; Optical tweezers; Viscoelasticity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors declare that they have no conflict of interest.
Figures





References
-
- Abu-Lail NI, Ohashi T, Clark RL, Erickson HP, Zauscher S (2006) Understanding the elasticity of fibronectin fibrils: unfolding strengths of FN-III and GFP domains measured by single molecule force spectroscopy. Matrix Biol J Int Soc Matrix Biol 25:175–184. 10.1016/j.matbio.2005.10.007 - DOI - PubMed
-
- Bowers SLK, Davis-Rodriguez S, Thomas ZM, Rudomanova V, Bacon WC, Beiersdorfer A, Ma Q, Devarajan P, Blaxall BC (2019) Inhibition of fibronectin polymerization alleviates kidney injury due to ischemia-reperfusion. Am J Physiol Renal Physiol 316:F1293–F1298. 10.1152/ajprenal.00117.2019 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

