Plasticity of Mouse Dorsal Root Ganglion Neurons by Innate Immune Activation Is Influenced by Electrophysiological Activity
- PMID: 39725852
- PMCID: PMC11671441
- DOI: 10.1111/jnc.16292
Plasticity of Mouse Dorsal Root Ganglion Neurons by Innate Immune Activation Is Influenced by Electrophysiological Activity
Abstract
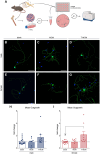
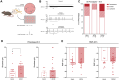
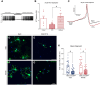
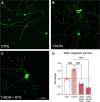
The complex relationship between inflammation, its effects on neuronal excitability and the ensuing plasticity of dorsal root ganglion (DRG) sensory neurons remains to be fully explored. In this study, we have employed a system of experiments assessing the impact of inflammatory conditioned media derived from activated immune cells on the excitability and activity of DRG neurons and how this relates to subsequent growth responses of these cells. We show here that an early phase of increased neuronal activity in response to inflammatory conditioned media is critical for the engagement of plastic processes and that neuronal excitability profiles are linked through time to the structural phenotype of individual neurons. Pharmacological blockade of neuronal activity was able to abolish the growth-promoting effects of inflammatory media. Our results suggest that targeting the activity of DRG neurons may provide a novel therapeutic avenue to manipulate their growth status and potential for plasticity in response to inflammation. Importantly, the same pharmacological blockade in vivo abolished pain responses in a mouse model of multiple sclerosis. While further studies are needed to fully elucidate the underlying mechanisms of the relationship between neural activity and growth status, a more complete understanding of this relationship may ultimately lead to the development of new treatments for neuropathic pain in disorders associated with heightened immune responses such as rheumatoid arthritis and multiple sclerosis.
Keywords: DRG; Kv7 channels; TNFα; electrophysiology; inflammation; neurite extension; pain; plasticity.
© 2024 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Benowitz, L. I. , and Popovich P. G.. 2011. “Inflammation and Axon Regeneration.” Current Opinion in Neurology 24, no. 6: 577–583. - PubMed
-
- Boettger, M. K. , Hensellek S., Richter F., et al. 2008. “Antinociceptive Effects of Tumor Necrosis Factor α Neutralization in a Rat Model of Antigen‐Induced Arthritis: Evidence of a Neuronal Target.” Arthritis & Rheumatism 58, no. 8: 2368–2378. - PubMed
-
- Bradke, F. , Fawcett J. W., and Spira M. E.. 2012. “Assembly of a New Growth Cone After Axotomy: The Precursor to Axon Regeneration.” Nature Reviews Neuroscience 13, no. 3: 183–193. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

