Collective Interactions of Quantum-Confined Excitons in Halide Perovskite Nanocrystal Superlattices
- PMID: 39725860
- PMCID: PMC11921029
- DOI: 10.1021/acsnano.4c12509
Collective Interactions of Quantum-Confined Excitons in Halide Perovskite Nanocrystal Superlattices
Abstract
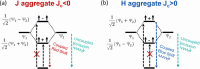
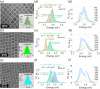
Collective optical properties can emerge from an ordered ensemble of emitters due to interactions between the individual units. Superlattices of halide perovskite nanocrystals exhibit collective light emission, influenced by dipole-dipole interactions between simultaneously excited nanocrystals. This coupling changes both the emission energy and rate compared to the emission of uncoupled nanocrystals. We demonstrate how quantum confinement governs the nature of the coupling between the nanocrystals in the ensemble. The extent of confinement is modified by controlling the nanocrystal size or by compositional control over the Bohr radius. In superlattices made of weakly confined nanocrystals, the collective emission is red-shifted with a faster emission rate, showing the key characteristics of superfluorescence. In contrast, the collective emission of stronger quantum-confined nanocrystals is blue-shifted with a slower emission rate. Both types of collective emission exhibit correlative multiphoton emission bursts, showing distinct photon bunching emission statistics. The quantum confinement changes the preferred alignment of transition dipoles within the nanocrystal and switches the relative dipole orientation between neighbors, resulting in opposite collective optical behaviors. Our results extend these collective effects to relatively high temperatures and provide a better understanding of exciton interactions and collective emission phenomena at the solid state.
Keywords: lead halide perovskites; nanocrystal coupling; nanocrystals; quantum confinement; superfluorescence; superlattices.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Dicke R. H. Coherence in spontaneous radiation processes. Phys. Rev. 1954, 93, 99–110. 10.1103/PhysRev.93.99. - DOI
-
- Gross M.; Haroche S. Superradiance: an essay on the theory of collective spontaneous emission. Phys. Rep. 1982, 93, 301–396. 10.1016/0370-1573(82)90102-8. - DOI
-
- Bonifacio R.; Lugiato L. A. Cooperative radiation processes in two-level systems: Superfluorescence. Phys. Rev. A 1975, 12, 587. 10.1103/PhysRevA.12.587. - DOI
-
- Skribanowitz N.; Herman P.; Macgillivray J. C.; Feld M. S. Observation of Dicke Superradiance in Optically Pumped HF Gas. Phys. Rev. Lett. 1973, 30, 309 10.1103/PhysRevLett.30.309. - DOI
LinkOut - more resources
Full Text Sources

