Hearing modulation affects Alzheimer's disease progression linked to brain inflammation: a study in mouse models
- PMID: 39725872
- PMCID: PMC11670416
- DOI: 10.1186/s10020-024-01040-1
Hearing modulation affects Alzheimer's disease progression linked to brain inflammation: a study in mouse models
Abstract
Background: Recent studies have identified hearing loss (HL) as a primary risk factor for Alzheimer's disease (AD) onset. However, the mechanisms linking HL to AD are not fully understood. This study explored the effects of drug-induced hearing loss (DIHL) on the expression of proteins associated with AD progression in mouse models.
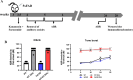
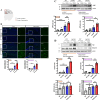
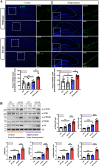
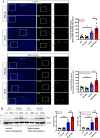
Methods: DIHL was induced in 5xFAD and Tg2576 mice aged 3 to 3.5 weeks using kanamycin (700 mg/kg, subcutaneous) and furosemide (600 mg/kg, intraperitoneal). The accumulation and expression of beta-amyloid (Aβ), ionized calcium-binding adaptor molecule 1 (Iba1), and glial fibrillary acidic protein (GFAP) were measured through immunohistochemistry and immunoblotting. Additionally, the expression of proteins involved in the mammalian target of rapamycin (mTOR) pathway, including downstream effectors p70 ribosomal S6 kinase (p70S6K) and S6, as well as proinflammatory cytokines, was analyzed.
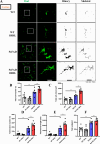
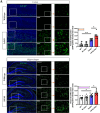
Results: Compared to control conditions, HL led to a significant increase in the accumulation of Aβ in the hippocampus and cortex. Elevated levels of neuroinflammatory markers, including Iba1 and GFAP, as well as proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α), were observed. Moreover, DIHL enhanced phosphorylation of mTOR, p70S6K, and S6, indicating activation of the mTOR pathway.
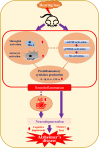
Conclusions: HL significantly increases Aβ accumulation in the brain. Furthermore, HL activates astrocytes and microglia, leading to increased neuroinflammation and thereby accelerating AD progression. These findings strongly suggest that HL contributes autonomously to neuroinflammation, highlighting the potential for early intervention in HL to reduce AD risk.
Keywords: Alzheimer’s disease; Drug-induced hearing loss; Glial fibrillary acidic protein; Iba1; Neuroinflammation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were approved by the Committee on the Ethics of Animal Experiments of Chonnam National University (CNUHIACUC-21052) and were performed in accordance with national and international guidelines for animal experimentation. Consents for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

