Activated DRP1 promotes mitochondrial fission and induces glycolysis in ATII cells under hyperoxia
- PMID: 39725939
- PMCID: PMC11670517
- DOI: 10.1186/s12931-024-03083-8
Activated DRP1 promotes mitochondrial fission and induces glycolysis in ATII cells under hyperoxia
Abstract
Backgroud: Recent studies have reported mitochondrial damage and metabolic dysregulation in BPD, but the changes in mitochondrial dynamics and glucose metabolic reprogramming in ATII cells and their regulatory relationship have not been reported.
Methods: Neonatal rats in this study were divided into model (FIO2:85%) and control (FIO2: 21%) groups. Lung tissues were extracted at 3, 7, 10 and 14 postnatal days and then conducted HE staining for histopathological observation. We assessed the expression of mitochondria dynamic associated proteins and glycolysis associated enzymes in lung tissues, primary ATII cells and RLE-6TN cells. Double immunofluorescence staining was used to confirm the co-localization of DRP1 and ATII cells. Real-time analyses of ECAR and OCR were performed with primary ATII cells using Seahorse XF96. ATP concentration was measured using an ATP kit. We treated RLE-6TN cells at 85% hyperoxia for 48 h with mitochondrial fission inhibitor Mdivi-1 to verify the role of DRP1 in regulating glucose metabolic reprogramming.
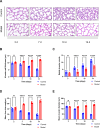
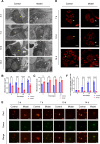
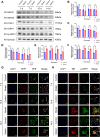
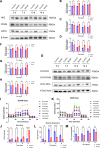
Findings: We found that hyperoxia causes ATII cells' mitochondrial morphological change. The expression of DRP1 and p-DRP1 increased in lung tissue and primary ATII cells of neonatal rats exposed to hyperoxia. Glycolysis related enzymes including PFKM, HK2, and LDHA were also increased. Hyperoxia inhibited ATP production in ATII cells. In RLE-6TN cells, we verified that the administration of Mdivi-1 could alleviate the enhancement of aerobic glycolysis and fragmentation of mitochondria caused by hyperoxia.
Interpretations: Hyperoxia exposure leads to increased mitochondrial fission in ATII cells and mediates the reprogramming of glucose metabolism via the DRP1 signaling pathway. Inhibiting the activation of DRP1 signaling pathway may be a promising therapeutic target for BPD.
Keywords: ATII cells; Bronchopulmonary dysplasia; DRP1 signaling pathway; Metabolic reprogramming; Mitochondrial fission.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The Ethics Committee of Animals at China Medical University approved and supervised all animal experiments (2023PS932K). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Katz TA, Vliegenthart RJS, Aarnoudse-Moens CSH, Leemhuis AG, Beuger S, Blok GJ, van Brakel MJM, van den Heuvel MEN, van Kempen A, Lutterman C, Rijpert M, Schiering IA, Ran NC, Visser F, Wilms J, van Kaam AH, Onland W. Severity of Bronchopulmonary Dysplasia and Neurodevelopmental Outcome at 2 and 5 years corrected age. J Pediatr. 2022;243:40–e4642. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

