Comparative profiling of white matter development in the human and mouse brain reveals volumetric deficits and delayed myelination in Angelman syndrome
- PMID: 39726042
- PMCID: PMC11670556
- DOI: 10.1186/s13229-024-00636-y
Comparative profiling of white matter development in the human and mouse brain reveals volumetric deficits and delayed myelination in Angelman syndrome
Abstract
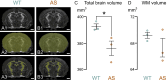
Background: Angelman syndrome (AS), a severe neurodevelopmental disorder resulting from the loss of the maternal UBE3A gene, is marked by changes in the brain's white matter (WM). The extent of WM abnormalities seems to correlate with the severity of clinical symptoms, but these deficits are still poorly characterized or understood. This study provides the first large-scale measurement of WM volume reduction in children with AS. Furthermore, we probed the possibility of underlying WM neuropathology by examining the progression of myelination in an AS mouse model.
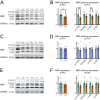
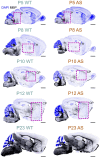
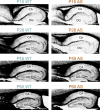
Methods: We conducted magnetic resonance imaging (MRI) on children with AS (n = 32) and neurotypical controls (n = 99) aged 0.5-12 years. In parallel, we examined myelination in postnatal Ube3a maternal-null mice (Ube3am-/p+; AS model), Ube3a paternal-null mice (Ube3am+/p-), and wildtype controls (Ube3am+/p+) using MRI, immunohistochemistry, western blotting, and electron microscopy.
Results: Our data revealed that AS individuals exhibit significant reductions in brain volume by ~ 1 year of age, and by 6-12 years of age WM is reduced by 26% and gray matter by 21%-approximately twice the reductions observed in the adult AS mouse model. Our AS mouse model saw a global delay in the onset of myelination, which normalized within days (likely corresponding to months or years in human development). This myelination delay is caused by the loss of UBE3A in neurons rather than UBE3A haploinsufficiency in oligodendrocytes. Interestingly, ultrastructural analyses did not reveal abnormalities in myelinated or unmyelinated axons.
Limitations: It is difficult to extrapolate the timing and duration of the myelination delay observed in AS model mice to individuals with AS.
Conclusions: This study reveals WM deficits as a hallmark in children with AS, demonstrating for the first time that these deficits are already apparent at 1 year of age. Parallel studies in a mouse model of AS show these deficits occur alongside the delayed onset of myelination, which results from the loss of neuronal (but not glial) UBE3A, though the causal relationship between these phenotypes remains to be determined. These findings emphasize the potential of WM as both a therapeutic target for interventions and a valuable biomarker for tracking the progression of AS and the effectiveness of potential treatments.
Keywords: Magnetic resonance imaging; Microcephaly; Myelin basic protein; Myelination; UBE3A; White matter.
© 2024. The Author(s).
Conflict of interest statement
Ethics approval and consent to participate: All procedures related to the care and treatment of animals followed institutional and NIH guidelines, and all animal use protocols were reviewed and approved by the UNC Institutional Animal Care and Use Committee. Parents of AS and NT individuals provided informed consent, and the institutional review board approved the research protocol. Competing interests: The authors declare no competing interests.
Figures











Update of
-
Comparative profiling of white matter development in the human and mouse brain reveals volumetric deficits and delayed myelination in Angelman syndrome.Res Sq [Preprint]. 2024 Aug 9:rs.3.rs-4681861. doi: 10.21203/rs.3.rs-4681861/v1. Res Sq. 2024. Update in: Mol Autism. 2024 Dec 26;15(1):54. doi: 10.1186/s13229-024-00636-y. PMID: 39149488 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- S10OD026796/GF/NIH HHS/United States
- 10MH124745/NHI
- S10 OD026796/OD/NIH HHS/United States
- P50 HD103573/HD/NICHD NIH HHS/United States
- R01 NS129914/NS/NINDS NIH HHS/United States
- award 702556/Simons Foundation Autism Research Initiative
- R01 NS131615/NS/NINDS NIH HHS/United States
- R01NS114086/NS/NINDS NIH HHS/United States
- T32 HD040127/HD/NICHD NIH HHS/United States
- NIH P50 HD103573-8084/Eunice Kennedy Shriver National Institute of Child Health and Human Development
- R01 MH120229/MH/NIMH NIH HHS/United States
- R56 NS097831/NS/NINDS NIH HHS/United States
- R01 NS114086/NS/NINDS NIH HHS/United States
- R01 HD093771/HD/NICHD NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- R01MH120229/MH/NIMH NIH HHS/United States
- S10 MH124745/MH/NIMH NIH HHS/United States
- NIH R01 HD093771/Eunice Kennedy Shriver National Institute of Child Health and Human Development
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

