Propofol Suppresses Ferroptosis via Modulating eNOS/NO Signaling Pathway to Improve Traumatic Brain Injury
- PMID: 39726103
- PMCID: PMC11671348
- DOI: 10.1002/brb3.70187
Propofol Suppresses Ferroptosis via Modulating eNOS/NO Signaling Pathway to Improve Traumatic Brain Injury
Abstract
Purpose: This study aims to explore the neuroprotective effect of propofol in improving traumatic brain injury (TBI) by inhibiting ferroptosis through the modulation of the endothelial nitric oxide (NO) synthase (eNOS)/NO signaling pathway.
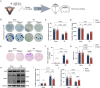
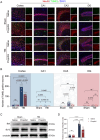
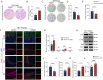
Methods: The GSE173975 dataset was used to analyze the differentially expressed genes between TBI and sham surgery control groups in the short and long term. A TBI model was established in 2-month-old male SPF C57BL/6 mice by impact exposure of the exposed dura mater. After the establishment of the TBI model, propofol (30 mg/kg) or saline was administered via intraperitoneal injection for intervention. Nissl staining and Perls staining were employed to assess neuronal function and iron deposition, respectively. Western blot technology was employed to detect the expression of proteins related to ferroptosis. Immunofluorescence staining of astrocytes and microglia was utilized to assess the neuroinflammatory response induced by TBI. The Morris water maze (MWM) and novel object recognition (NOR) tests were employed to assess cognitive dysfunction induced by TBI.
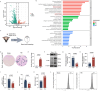
Findings: Bioinformatics analysis revealed aberrant gene expression associated with iron transport, neuronal death, and inflammatory response in the initial stages of TBI. Long-term abnormalities were predominantly linked to genes involved in inflammatory response. Perls staining and protein expression analysis confirmed the occurrence of iron deposition and ferroptosis following TBI. Propofol treatment significantly reduced iron deposition and ferroptosis induced by TBI. Nissl staining demonstrated enhanced neuronal function, while TUNEL staining indicated reduced neuronal apoptosis. Immunofluorescence analysis demonstrated that propofol significantly reduced the proliferation of astrocytes and activation of microglia induced by TBI in the long term. The results of MWM and NOR tests indicated that propofol significantly improved the long-term cognitive dysfunction induced by TBI. Propofol exerts neuroprotective effects by increasing the expression of eNOS protein and the content of NO. The neuroprotective effects of propofol can be reversed by the eNOS inhibitor L-NAME.
Conclusion: Propofol significantly improves the prognosis of TBI by inhibiting ferroptosis through the modulation of the eNOS/NO signaling pathway. The study results provide a scientific basis for the clinical use of propofol as a neuroprotective agent and offer a new direction for the development of new treatment strategies for TBI.
Keywords: eNOS; ferroptosis; nitric oxide; propofol; traumatic brain injury.
© 2024 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Propofol alleviates traumatic brain injury through regulating Th17/Treg balance by activation of the AMPK/SIRT1 pathway.Toxicol Mech Methods. 2025 Jul;35(6):644-654. doi: 10.1080/15376516.2025.2481893. Epub 2025 Apr 2. Toxicol Mech Methods. 2025. PMID: 40172234
-
Salidroside Exerts Neuroprotective Effects via Inhibiting Ferroptosis in Mice with Traumatic Brain Injury.Neurochem Res. 2025 Jun 21;50(4):204. doi: 10.1007/s11064-025-04430-x. Neurochem Res. 2025. PMID: 40542883
-
Trehalose Inhibits ferroptosis Through Activating SIRT3/SOD2 Signaling Axis and Alleviates Brain Injury After Traumatic Brain Injury.Neurochem Res. 2025 Jan 11;50(1):78. doi: 10.1007/s11064-024-04330-6. Neurochem Res. 2025. PMID: 39798057
-
Metabolic dysfunction contributes to mood disorders after traumatic brain injury.Ageing Res Rev. 2025 Feb;104:102652. doi: 10.1016/j.arr.2024.102652. Epub 2024 Dec 31. Ageing Res Rev. 2025. PMID: 39746403 Review.
-
Novel pharmaceutical treatments for minimal traumatic brain injury and evaluation of animal models and methodologies supporting their development.J Neurosci Methods. 2016 Oct 15;272:69-76. doi: 10.1016/j.jneumeth.2016.02.002. Epub 2016 Feb 8. J Neurosci Methods. 2016. PMID: 26868733 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

