Support-Free, Connected Core-Shell Nanoparticle Catalysts Synthesized via a Low-Temperature Process for Advanced Oxygen Reduction Performance
- PMID: 39726238
- PMCID: PMC11831485
- DOI: 10.1002/advs.202408614
Support-Free, Connected Core-Shell Nanoparticle Catalysts Synthesized via a Low-Temperature Process for Advanced Oxygen Reduction Performance
Abstract
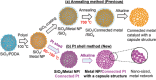
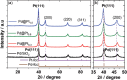
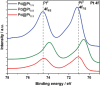
Nanostructured Pt-based catalysts have attracted considerable attention for fuel-cell applications. This study introduces a novel one-pot and low-temperature polyol approach for synthesizing support-free, connected nanoparticles with non-Pt metal cores and Pt shells. Unlike conventional heat treatment methods, the developed support-free and Fe-free connected Pdcore@Ptshell (Pd@Pt) nanoparticle catalyst possesses a stable nanonetwork structure with a high surface area. This approach can precisely control the atomic-level structure of the Pt shell on the Pd core at a low deposition temperature. The optimized Pd@Pt catalyst with a Pt/Pd atomic ratio of 0.8 and a Pt shell thickness of 1.1 nm exhibits a threefold improvement in oxygen reduction reaction (ORR) mass activity compared to that of commercial carbon-supported Pt nanoparticle catalyst (Pt/C). Durability evaluation demonstrated 100% retention of specific activity after 10,000 load cycles, owing to the stable nanonetwork and uniform coverage of the Pt shell. In addition, the support-free, connected core-shell nanoparticle catalyst overcomes the carbon corrosion issues commonly associated with conventional carbon-supported catalysts while simultaneously improving both ORR activity and load cycle durability. These findings highlight the potential of this innovative approach to develop support-free catalysts for polymer electrolyte fuel cells and other energy devices.
Keywords: Pt shell; connected core–shell nanoparticles; oxygen reduction reaction; polymer electrolyte fuel cell; support‐free catalyst.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Staffell I., Scamman D., Velazquez Abad A., Balcombe P., Dodds P. E., Ekins P., Shah N., Ward K. R., Energy Environ. Sci. 2019, 12, 463.
-
- Zhu F., Luo L., Wu A., Wang C., Cheng X., Shen S., Ke C., Yang H., Zhang J., ACS Appl. Mater. Interfaces 2020, 12, 26076. - PubMed
-
- Pollet B. G., Kocha S. S., Staffell I., Curr. Opin. Electrochem. 2019, 16, 90.
-
- Zhao J., Tu Z., Chan S. H., J. Power Sources 2021, 488, 229434.
-
- Wang Y., Wang D., Li Y., SmartMat 2021, 2, 56.
Grants and funding
LinkOut - more resources
Full Text Sources
