Ranking Single Fluorescent Protein-Based Calcium Biosensor Performance by Molecular Dynamics Simulations
- PMID: 39726324
- PMCID: PMC11733952
- DOI: 10.1021/acs.jcim.4c01478
Ranking Single Fluorescent Protein-Based Calcium Biosensor Performance by Molecular Dynamics Simulations
Abstract
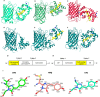
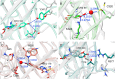
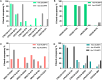
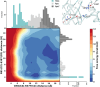
Genetically encoded fluorescent biosensors (GEFBs) have become indispensable tools for visualizing biological processes in vivo. A typical GEFB is composed of a sensory domain (SD) that undergoes a conformational change upon ligand binding or enzymatic reaction; the SD is genetically fused with a fluorescent protein (FP). The changes in the SD allosterically modulate the chromophore environment whose spectral properties are changed. Single fluorescent (FP)-based biosensors, a subclass of GEFBs, offer a simple experimental setup; they are easy to produce in living cells, structurally stable, and simple to use due to their single-wavelength operation. However, they pose a significant challenge for structure optimization, especially concerning the length and residue content of linkers between the FP and SD, which affect how well the chromophore responds to conformational change in the SD. In this work, we use all-atom molecular dynamics simulations to analyze the dynamic properties of a series of calmodulin-based calcium biosensors, all with different FP-SD interaction interfaces and varying degrees of calcium binding-dependent fluorescence change. Our results indicate that biosensor performance can be predicted based on distribution of water molecules around the chromophore and shifts in hydrogen bond occupancies between the ligand-bound and ligand-free sensor structures.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Allosteric modulation of fluorescence revealed by hydrogen bond dynamics in a genetically encoded maltose biosensor.Proteins. 2024 Aug;92(8):923-932. doi: 10.1002/prot.26688. Epub 2024 Apr 4. Proteins. 2024. PMID: 38572606
-
Development of an miRFP680-Based Fluorescent Calcium Ion Biosensor Using End-Optimized Transposons.ACS Sens. 2024 Jun 28;9(6):3394-3402. doi: 10.1021/acssensors.4c00727. Epub 2024 Jun 1. ACS Sens. 2024. PMID: 38822813 Free PMC article.
-
Excited-state structural dynamics of a dual-emission calmodulin-green fluorescent protein sensor for calcium ion imaging.Proc Natl Acad Sci U S A. 2014 Jul 15;111(28):10191-6. doi: 10.1073/pnas.1403712111. Epub 2014 Jul 1. Proc Natl Acad Sci U S A. 2014. PMID: 24987121 Free PMC article.
-
Genetically encoded biosensors based on innovative scaffolds.Int J Biochem Cell Biol. 2020 Aug;125:105761. doi: 10.1016/j.biocel.2020.105761. Epub 2020 Jun 3. Int J Biochem Cell Biol. 2020. PMID: 32504671 Review.
-
Structure- and mechanism-guided design of single fluorescent protein-based biosensors.Nat Chem Biol. 2021 May;17(5):509-518. doi: 10.1038/s41589-020-00718-x. Epub 2021 Feb 8. Nat Chem Biol. 2021. PMID: 33558715 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

