Evaluation the protective role of baicalin against H2O2-driven oxidation, inflammation and apoptosis in bovine mammary epithelial cells
- PMID: 39726580
- PMCID: PMC11669685
- DOI: 10.3389/fvets.2024.1504887
Evaluation the protective role of baicalin against H2O2-driven oxidation, inflammation and apoptosis in bovine mammary epithelial cells
Abstract
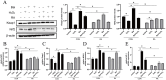
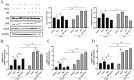
Mastitis is one of the most common diseases in dairy farms. During the perinatal period, the bovine mammary epithelial cells (BMECs) of High-yielding dairy cows accelerate metabolism and produce large amounts of reactive oxygen species (ROS). It is one of the primary causes of mastitis and will lead to the breakdown of redox balance, which will induce oxidative stress, inflammation, and apoptosis. Baicalin is a flavonoid substance extracted from the root of natural plant Scutellaria baicalensis, which has anti-inflammatory, anti-oxidant, anti-viral and other biological functions. In this research, hydrogen peroxide (H2O2) was used to construct a mastitis oxidative stress model, and relevant mechanisms were analyzed by immunofluorescence techniques, qRT-PCR and Western Blot to explore how baicalin affects BMECs' oxidative stress and inflammation caused by H2O2, as well as to provide new perspectives on the combined application of baicalin in the prevention and treatment of mastitis. The results demonstrated that baicalin treatment could reduce the accumulation of H2O2-induced intracellular ROS and decrease the expression of inflammatory cytokines Tumor Necrosis Factor-α (TNF-α), interleukin 6 (IL-6), interleukin-1β (IL-1β) and the apoptosis rate. The inhibitory effect of baicalin on H2O2-induced intracellular ROS accumulation and the expression of inflammatory cytokines and apoptotic factors in BMECs was blocked by pretreatment with the Nuclear factor erythroid 2-related factor 2 (Nrf2) inhibitor retinoic acid (RA) prior to H2O2 and/or baicalin treatment. In summary, baicalin could served as a natural antioxidant agent to regulate cell apoptosis through its anti-inflammatory, antioxidant and anti-apoptotic effects to combat BMECs damage caused by H2O2.
Keywords: apoptosis; baicalin; bovine mammary epithelial cells (BMECs); inflammation; nuclear factor erythroid 2-related factor 2 (Nrf2); oxidative stress.
Copyright © 2024 Kong, Wang, Guo, Yang, Lian, Gao, Zhang and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Piras F, Sogos V, Pollastro F, Appendino G, Rosa A. Arzanol, a natural phloroglucinol α-pyrone, protects HaCaT keratinocytes against H2O2-induced oxidative stress, counteracting cytotoxicity, reactive oxygen species generation, apoptosis, and mitochondrial depolarization. J Appl Toxicol. (2024) 44:720–32. 10.1002/jat.4570 - DOI - PubMed
LinkOut - more resources
Full Text Sources

