Immunogenicity and protective efficacy of recombinant chimeric antigens based on surface proteins of Toxoplasma gondii
- PMID: 39726608
- PMCID: PMC11670819
- DOI: 10.3389/fimmu.2024.1480349
Immunogenicity and protective efficacy of recombinant chimeric antigens based on surface proteins of Toxoplasma gondii
Abstract
Introduction: Toxoplasmosis is caused by the opportunistic, cosmopolitan protozoan Toxoplasma gondii is one of the most common parasitoses in the world. This parasite can pose a threat to people with immunodeficiency but also to the fetus, since the invasion can lead to miscarriages. Moreover, this parasite can contribute to economic losses in livestock farming. These problems lead to the implementation of new, safe solutions for the development of effective toxoplasmosis immunoprophylaxis.
Methods: In this work, newly produced recombinant trivalent chimeric proteins of T. gondii, based on SAG1-SAG2 recombinant chimeric antigen that differ in one terminal antigenic component, were tested in terms of their ability to induce an effective post-vaccination response. Antigens were tested in vitro to assess their ability to elicit APC cells response and further mice of the C3H/HeOuJ strain were immunized using those antigens, to evaluate their immunogenicity and immunoprotective effect in vivo. Two weeks after the last dose mice were either sacrificed to assess selected parameters of the immune response or infected with T. gondii DX strain to determine the degree of protection one month later.
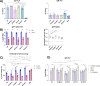
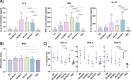
Results: The results of serological tests revealed a high level of serum IgG antibodies specific for the native T. gondii TLA antigens. TLA-stimulated splenocytes produced cytokines that are important in inhibiting protozoal invasion. Additionally, CD3+ CD4+ and CD3+ CD8+ T cell subpopulations of splenocytes were analysed by flow cytometry. One month after experimental infection mice were sacrificed, and their brains were isolated to count T. gondii tissue cyst. Immunization of mice with recombinant trivalent chimeric proteins of T. gondii resulted in reduction of tissue cyst burden rates reaching even 74%.
Discussion: The obtained results demonstrate strong immunogenicity of the studied proteins and will allow to select candidates for further research aimed at increasing the immunoprotective properties of experimental vaccines against toxoplasmosis based on T. gondii chimeric antigens.
Keywords: T. gondii experimental vaccine; Toxoplasma gondii; immunoprotection; murine experimental model; recombinant chimeric antigen.
Copyright © 2024 Chyb, Ferra, Kawka, Skwarecka, Dziadek and Gatkowska.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Evaluation of three recombinant multi-antigenic vaccines composed of surface and secretory antigens of Toxoplasma gondii in murine models of experimental toxoplasmosis.Vaccine. 2011 Jan 17;29(4):821-30. doi: 10.1016/j.vaccine.2010.11.002. Epub 2010 Nov 16. Vaccine. 2011. PMID: 21087690
-
The Development of Toxoplasma gondii Recombinant Trivalent Chimeric Proteins as an Alternative to Toxoplasma Lysate Antigen (TLA) in Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Immunoglobulin G (IgG) in Small Ruminants.Int J Mol Sci. 2024 Apr 16;25(8):4384. doi: 10.3390/ijms25084384. Int J Mol Sci. 2024. PMID: 38673969 Free PMC article.
-
Evaluation of protective efficacy of recombinant Toxoplasma gondii DDX39 protein vaccine against acute and chronic T. gondii infection in mice.Acta Trop. 2024 Dec;260:107442. doi: 10.1016/j.actatropica.2024.107442. Epub 2024 Oct 24. Acta Trop. 2024. PMID: 39461580
-
Toxoplasma gondii surface antigen 1 (SAG1) as a potential candidate to develop vaccine against toxoplasmosis: A systematic review.Comp Immunol Microbiol Infect Dis. 2020 Apr;69:101414. doi: 10.1016/j.cimid.2020.101414. Epub 2020 Jan 7. Comp Immunol Microbiol Infect Dis. 2020. PMID: 31958746
-
A systematic review on the role of GRA proteins of Toxoplasma gondii in host immunization.J Microbiol Methods. 2019 Oct;165:105696. doi: 10.1016/j.mimet.2019.105696. Epub 2019 Aug 20. J Microbiol Methods. 2019. PMID: 31442457
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

