Quantifying CSF Dynamics disruption in idiopathic normal pressure hydrocephalus using phase lag between transmantle pressure and volumetric flow rate
- PMID: 39726610
- PMCID: PMC11671130
- DOI: 10.1016/j.brain.2024.100101
Quantifying CSF Dynamics disruption in idiopathic normal pressure hydrocephalus using phase lag between transmantle pressure and volumetric flow rate
Abstract
Background and purpose: Idiopathic normal pressure hydrocephalus (iNPH) is a cerebrospinal fluid (CSF) dynamics disorder as evidenced by the delayed ascent of radiotracers over the cerebral convexity on radionuclide cisternography. However, the exact mechanism causing this disruption remains unclear. Elucidating the pathophysiology of iNPH is crucial, as it is a treatable cause of dementia. Improving the diagnosis and treatment prognosis rely on the better understanding of this disease. In this study, we calculated the pulsatile transmantle pressure and investigated the phase lag between this pressure and the volumetric CSF flow rate as a novel biomarker of CSF dynamics disruption in iNPH.
Methods: 44 iNPH patients and 44 age- and sex-matched cognitively unimpaired (CU) control participants underwent MRI scans on a 3T Siemens scanner. Pulsatile transmantle pressure was calculated analytically and computationally using volumetric CSF flow rate, cardiac frequency, and aqueduct dimensions as inputs. CSF flow rate through the aqueduct was acquired using phase-contrast MRI. The aqueduct length and radius were measured using 3D T1-weighted anatomical images.
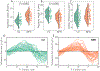
Results: Peak pressure amplitudes and the pressure load (integrated pressure exerted over a cardiac cycle) were similar between the groups, but the non-dimensionalized pressure load (adjusted for anatomical factors) was significantly lower in the iNPH group ( , Welch's t-test). The phase lag between the pressure and the flow rate, arising due to viscous drag, was significantly higher in the iNPH group ( ).
Conclusion: The increased phase lag is a promising new biomarker for quantifying CSF dynamics dysfunction in iNPH.
Statement of significance: The exact mechanism causing the disruption of CSF circulation in idiopathic normal pressure hydrocephalus (iNPH) remains unclear. Elucidating the pathophysiology of iNPH is crucial, as it is a treatable cause of dementia. In this study, we provided an analytical and a computational method to calculate the pulsatile transmantle pressure and the phase lag between the pressure and the volumetric CSF flow rate across the cerebral aqueduct. The phase lag was significantly higher in iNPH patients than in controls and may serve as a novel biomarker of CSF dynamics disruption in iNPH.
Keywords: Csf dynamics disorder; Normal pressure hydrocephalus; Phase lag; Phase-contrast mri; Transmantle pressure; Womersley number.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



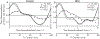


References
-
- Patten DH, Benson DF, Diagnosis of normal-pressure hydrocephalus by RISA cisternography, J. Nucl. Med 9 (8) (1968) 457–461. - PubMed
-
- Luetmer PH, et al. , Measurement of cerebrospinal fluid flow at the cerebral aqueduct by use of phase-contrast magnetic resonance imaging: technique validation and utility in diagnosing idiopathic normal pressure hydrocephalus (in eng), Neurosurg. 50 (3) (2002) 534–543, 10.1097/00006123-200203000-00020. discussion 543–4, Mar. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
