Developmental origins of natural sound perception
- PMID: 39726626
- PMCID: PMC11669913
- DOI: 10.3389/fpsyg.2024.1474961
Developmental origins of natural sound perception
Abstract
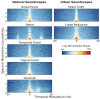
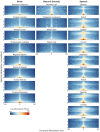
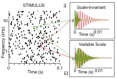
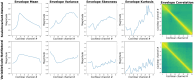
Infants are exposed to a myriad of sounds early in life, including caregivers' speech, songs, human-made and natural (non-anthropogenic) environmental sounds. While decades of research have established that infants have sophisticated perceptual abilities to process speech, less is known about how they perceive natural environmental sounds. This review synthesizes current findings about the perception of natural environmental sounds in the first years of life, emphasizing their role in auditory development and describing how these studies contribute to the emerging field of human auditory ecology. Some of the existing studies explore infants' responses to animal vocalizations and water sounds. Infants demonstrate an initial broad sensitivity to primate vocalizations, which narrows to human speech through experience. They also show early recognition of water sounds, with preferences for natural over artificial water sounds already at birth, indicating an evolutionary ancient sensitivity. However, this ability undergoes refinement with age and experience. The few studies available suggest that infants' auditory processing of natural sounds is complex and influenced by both genetic predispositions and exposure. Building on these existing results, this review highlights the need for ecologically valid experimental paradigms that better represent the natural auditory environments humans evolved in. Understanding how children process natural soundscapes not only deepens our understanding of auditory development but also offers practical insights for advancing environmental awareness, improving auditory interventions for children with hearing loss, and promoting wellbeing through exposure to natural sounds.
Keywords: animal vocalizations; auditory development; children; environmental sounds; human auditory ecology; infants; natural soundscapes; water sounds.
Copyright © 2024 Polver, Miller-Viacava, Fraticelli, Gervain and Lorenzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

