A Pharmacokinetic/Toxicodynamic Model of Cisplatin Nephrotoxicity Using the Kidney Injury Molecule-1 Biomarker
- PMID: 39726772
- PMCID: PMC11671133
- DOI: 10.47739/pharmacology1184
A Pharmacokinetic/Toxicodynamic Model of Cisplatin Nephrotoxicity Using the Kidney Injury Molecule-1 Biomarker
Abstract
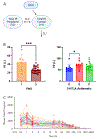
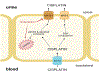
Cisplatin is a platinum-based chemotherapeutic that causes acute kidney injury in over 30% of patients. The aim of this study was to develop a population pharmacokinetic/toxicodynamic (PKTD) model of cisplatin-induced kidney injury that incorporated plasma total platinum and urinary kidney injury molecule-1 (KIM-1) concentrations. Cancer patients receiving their first or second round of cisplatin-containing chemotherapy (n=39) were prospectively randomized to a 5-HT3 antagonist (5-HT3A) antiemetic (ondansetron, granisetron, or palonosetron) and had blood and urine collected over 10 days. Plasma concentrations of total platinum and urinary concentrations of KIM-1 were used in the development of a nonlinear mixed effect population PKTD model using Phoenix® NLME (v8.3, Certara Inc.). A stepwise search was used to test potential covariates that influenced PKTD parameters. A two-compartment model best described the plasma total platinum concentration vs. time data and was expanded to an effect compartment PKTD model incorporating urinary KIM-1 concentrations. Significant covariate effects for the PKTD model included previous cisplatin exposure on the volume of the central compartment (V1), 5-HT3A antiemetic treatment on the volume of the peripheral compartment (V2), and baseline urinary KIM-1 levels on the maximum effect (Emax) parameter. The model demonstrated that ondansetron-treated subjects had a 163% increase in exposure to plasma total platinum, a 94% increase in urinary KIM-1 maximum concentrations, and a 235% increase in total urinary KIM-1 excretion compared to palonosetron-treated subjects, suggesting that palonosetron may be a preferred 5-HT3A to reduce the risk of cisplatin-induced kidney injury.
Keywords: 5-HT3 Antagonist; Cisplatin; Kidney Injury; Nephrotoxicity; Pharmacodynamics; Pharmacokinetics; Toxicodynamics.
Conflict of interest statement
CONFLICT OF INTEREST The authors declare no conflicts of interest. Lauren M. Aleksunes is a member of the scientific advisory board of RenaSym® Simulations Plus.
Figures
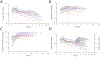





References
Grants and funding
LinkOut - more resources
Full Text Sources
