Salivary oxytocin and amygdalar alterations in functional neurological disorders
- PMID: 39726815
- PMCID: PMC11670354
- DOI: 10.1093/braincomms/fcae455
Salivary oxytocin and amygdalar alterations in functional neurological disorders
Abstract
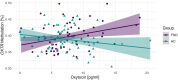
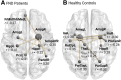
Individuals diagnosed with functional neurological disorder experience abnormal movement, gait, sensory processing or functional seizures, for which research into the pathophysiology identified psychosocial contributing factors as well as promising biomarkers. Recent pilot studies suggested that (epi-)genetic variants may act as vulnerability factors, for example, on the oxytocin pathway. This study set out to explore endogenous oxytocin hormone levels in saliva in a cohort of 59 functional neurological disorder patients and 65 healthy controls comparable in sex and age. First, we examined the association between salivary oxytocin levels with the genetic allelic variant (rs53576) of the oxytocin receptor gene (OXTR), its epigenetic changes indicated by methylation rates, and clinical variables-including childhood trauma. Second, due to previously reported effects of oxytocin changing the volume and functional connectivity of the amygdala, as well as the known involvement of the amygdala in the pathophysiology of functional neurological disorders, we further looked at both structural and functional imaging of the amygdala. While patients did not significantly differ from healthy control in their peripheral oxytocin levels, there was a specific interaction of OXTR methylation and peripheral oxytocin dependent on group: higher methylation rates correlated with higher salivary oxytocin in patients only, while this was not the case in healthy control [F(1109) = 8.92, P = 0.003, d = 0.541]. Moreover, patients with the AA-genotype (minor allele) of the rs53576 genetic variant of the OXTR gene presented with higher OXTR methylation levels [F(2106) = 10.25, P < 0.0001, d = 0.58]. Lastly, amygdalar connectivity to the hippocampus, the posterior cingulate cortex, the inferior parietal cortex and the inferior temporal cortex as well as smaller amygdalar volume were correlated to peripheral oxytocin levels in patients only [F(2,38) = 5.36, P = 0.025, d = 0.431], but not in healthy control. No significant interactions with childhood trauma were identified. Our study revealed a significant interplay between peripheral oxytocin and OXTR methylation in patients only, potentially influenced by genotype. One could hypothesize that higher peripheral oxytocin denotes a compensatory mechanisms for the increased methylation of the OXTR, which might affect amygdalar functional connectivity. These findings help to further understand underlying pathophysiological mechanisms, considering oxytocin's involvement in functional patients and could offer a potential site of treatment for future studies.
Keywords: OXTR; amygdala; conversion disorder; epigenetics; rs53576.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

Similar articles
-
Childhood emotional neglect and oxytocin receptor variants: Association with limbic brain volumes.World J Biol Psychiatry. 2020 Sep;21(7):513-528. doi: 10.1080/15622975.2019.1584331. Epub 2019 Apr 2. World J Biol Psychiatry. 2020. PMID: 30806136
-
Methylation of the oxytocin receptor gene in clinically depressed patients compared to controls: The role of OXTR rs53576 genotype.J Psychiatr Res. 2015 Jun;65:9-15. doi: 10.1016/j.jpsychires.2015.03.012. Epub 2015 Mar 24. J Psychiatr Res. 2015. PMID: 25890851
-
Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety.Neuropsychopharmacology. 2015 May;40(6):1528-38. doi: 10.1038/npp.2015.2. Epub 2015 Jan 7. Neuropsychopharmacology. 2015. PMID: 25563749 Free PMC article.
-
Review of eating disorders and oxytocin receptor polymorphisms.J Eat Disord. 2021 Jul 13;9(1):85. doi: 10.1186/s40337-021-00438-0. J Eat Disord. 2021. PMID: 34256847 Free PMC article. Review.
-
Intranasal oxytocin and OXTR genotype effects on resting state functional connectivity: A systematic review.Neurosci Biobehav Rev. 2018 Dec;95:17-32. doi: 10.1016/j.neubiorev.2018.09.011. Epub 2018 Sep 19. Neurosci Biobehav Rev. 2018. PMID: 30243577
References
-
- Aybek S, Perez DL. Diagnosis and management of functional neurological disorder. BMJ. 2022;376:o64. - PubMed
-
- Ludwig L, Pasman JA, Nicholson T, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: Systematic review and meta-analysis of case-control studies. Lancet Psychiatry. 2018;5(4):307–320. - PubMed
-
- Conejero I, Collombier L, Lopez-Castroman J, et al. Association between brain metabolism and clinical course of motor functional neurological disorders. Brain. 2022;145(9):3264–3273. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
