FSH/LH co-stimulation in Advanced Maternal Age (AMA) and hypo-responder patients - Arabian gulf delphi consensus group
- PMID: 39726844
- PMCID: PMC11669953
- DOI: 10.3389/fendo.2024.1506332
FSH/LH co-stimulation in Advanced Maternal Age (AMA) and hypo-responder patients - Arabian gulf delphi consensus group
Abstract
Background: In a global effort to assess expert perspectives on the use of recombinant gonadotropins, recombinant human luteinizing hormone (r-hLH) and recombinant human follicle-stimulating hormone (r-hFSH), a consensus meeting was held in Dubai. The key aim was to address three critical questions: What are the factors that influence follicle response to gonadotropins? Which categories of patients are most likely to benefit from LH supplementation? And what are the optimal management strategies for these patients?
Methods: A panel of thirty-six experts reviewed and refined the initial statements and references proposed by the Scientific Coordinator. Consensus was defined as agreement or disagreement by more than two-thirds (66%) of the panel members for each statement.
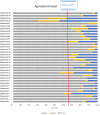
Results: Thirty-five statements were formulated, of which thirty-one reached consensus. For patients with Hypo-Response to Gonadotropin Stimulation (20 statements), all identified risk factors, including advanced age, high BMI, and chronic conditions, achieved unanimous agreement. Diagnostic approaches, such as the inclusion of POSEIDON criteria and hormone level monitoring, were endorsed by the majority, with over 90% agreement. Management strategies, particularly individualized stimulation protocols and optimized scheduling, garnered broad consensus, with only one statement falling short of the threshold. Additionally, in cases of severe FSH and LH deficiency, combining r-hFSH with r-hLH was found to improve pregnancy rates and cost efficiency compared to human menopausal gonadotropin (hMG). For patients with Advanced Maternal Age (AMA) (15 statements), there was strong agreement on the use of oral contraceptive pills and estrogen priming. Recommendations concerning antagonist protocols and dosing of r-hLH and r-hFSH also achieved high levels of consensus. Significant agreement supported r-hLH supplementation and a tailored approach to luteal phase support. However, there were mixed opinions on the route of progesterone administration, with some experts expressing neutral or disagreeing views. Despite these differences, unanimous consensus was reached on markers of treatment success, particularly live birth rates, pregnancy rates, and embryo development, underscoring the importance of these outcomes in evaluating treatment efficacy.
Conclusion: This consensus provides a practical clinical perspective to a wide range of global professionals on the strategies employed during key phases of Assisted Reproductive Technology (ART) treatment. To further improve outcomes, incorporating additional clinical insights on ART approaches, alongside existing guidelines and policies, may offer valuable guidance for optimizing patient care.
Keywords: FSH; LH; advanced; gonadotrophins; poor ovarian reserve; poor responders.
Copyright © 2024 Awwad, Peramo, Elgeyoushi, Melado, Salame, Chawla, Jibrel, Detho, Al Rumaih, Tomsu, Fahim, Abd-ElGawad, Fouad and Humaidan.
Conflict of interest statement
This project was sponsored and funded by Merck Serono Middle East FZ-LTD, Dubai, United Arab Emirates, an affiliate of Merck KGaA. The sponsor was involved early in the process, defining the overarching topic to be discussed, but did not participate in the development of the statements or in any of the meetings or discussions involved in developing the Delphi consensus. The statements were, therefore, developed independently of the industry sponsor. The authors from Merck Serono Middle East, Dubai, United Arab Emirates, were only involved in the development of the manuscript, critically revising it for important intellectual content, especially in the Introduction, Results and Discussion sections, but could not alter the consensus statements in any way. Authors AF and KF were employed by the company Merck Serono Middle East FZ-LTD, Dubai, United Arab Emirates, an affiliate of Merck KGaA.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources