Key Pathophysiological Role of Skeletal Muscle Disturbance in Post COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Accumulated Evidence
- PMID: 39727052
- PMCID: PMC11671797
- DOI: 10.1002/jcsm.13669
Key Pathophysiological Role of Skeletal Muscle Disturbance in Post COVID and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Accumulated Evidence
Abstract
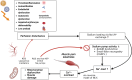
Background: Recent studies provide strong evidence for a key role of skeletal muscle pathophysiology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). In a 2021 review article on the pathophysiology of ME/CFS, we postulated that hypoperfusion and ischemia can result in excessive sodium and calcium overload in skeletal muscles of ME/CFS patients to cause mitochondrial damage. Since then, experimental evidence has been provided that supports this concept.
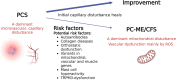
Methods: We collect, summarize and discuss the current state of knowledge for the key role of skeletal muscle pathophysiology. We try to explain which risk factors and mechanisms are responsible for a subgroup of patients with post COVID syndrome (PCS) to develop ME/CFS (PC-ME/CFS).
Results: Mitochondrial dysfunction is a long-held assumption to explain cardinal symptoms of ME/CFS. However, mitochondrial dysfunction could not be convincingly shown in leukocytes. By contrast, recent studies provide strong evidence for mitochondrial dysfunction in skeletal muscle tissue in ME/CFS. An electron microscopy study could directly show damage of mitochondria in skeletal muscle of ME/CFS patients with a preferential subsarcolemmal localization but not in PCS. Another study shows signs of skeletal muscle damage and regeneration in biopsies taken one day after exercise in PC-ME/CFS. The simultaneous presence of necroses and signs of regeneration supports the concept of repeated damage. Other studies correlated diminished hand grip strength (HGS) with symptom severity and prognosis. A MRI study showed that intracellular sodium in muscles of ME/CFS patients is elevated and that levels correlate inversely with HGS. This finding corroborates our concept of sodium and consecutive calcium overload as cause of muscular and mitochondrial damage caused by enhanced proton-sodium exchange due to anaerobic metabolism and diminished activity of the sodium-potassium-ATPase. The histological investigations in ME/CFS exclude ischemia by microvascular obstruction, viral presence or immune myositis. The only known exercise-induced mechanism of damage left is sodium induced calcium overload. If ionic disturbance and mitochondrial dysfunction is severe enough the patient may be captured in a vicious circle. This energy deficit is the most likely cause of exertional intolerance and post exertional malaise and is further aggravated by exertion.
Conclusion: Based on this pathomechanism, future treatment approaches should focus on normalizing the cause of ionic disbalance. Current treatment strategies targeting hypoperfusion have the potential to improve the dysfunction of ion transporters.
Keywords: long COVID; mitochondrial damage; myalgic encephalomyelitis/chronic fatigue syndrome; post–COVID‐19; skeletal muscle; vascular dysfunction.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Charité holds patents for the use of beta‐adrenergic receptor antibodies in the diagnosis of CFS and for soluble guanylate cyclase activators for treating chronic vascular dysfunction. Carmen Scheibenbogen received honoraria from Celltrend, Roche and Bayer for consultation.
Klaus J. Wirth is the managing director of Mitodicure GmbH, a startup developing a small molecule therapeutic for the treatment of ME/CFS.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

