Prediction of Central Post-Stroke Pain by Quantitative Sensory Testing
- PMID: 39727056
- PMCID: PMC11831871
- DOI: 10.1002/ana.27138
Prediction of Central Post-Stroke Pain by Quantitative Sensory Testing
Abstract
Objective: Among patients with acute stroke, we aimed to identify those who will later develop central post-stroke pain (CPSP) versus those who will not (non-pain sensory stroke [NPSS]) by assessing potential differences in somatosensory profile patterns and evaluating their potential as predictors of CPSP.
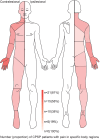
Methods: In a prospective longitudinal study on 75 acute stroke patients with somatosensory symptoms, we performed quantitative somatosensory testing (QST) in the acute/subacute phase (within 10 days) and on follow-up visits for 12 months. Based on previous QST studies, we hypothesized that QST values of cold detection threshold (CDT) and dynamic mechanical allodynia (DMA) would differ between CPSP and NPSS patients before the onset of pain. Mann-Whitney U-tests and mixed analysis of variances with Bonferroni corrections were performed to compare z-normalized QST scores between both groups.
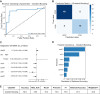
Results: In total, 26 patients (34.7%) developed CPSP. In the acute phase, CPSP patients showed contralesional cold hypoesthesia compared to NPSS patients (p = 0.04), but no DMA differences. Additional exploratory analysis showed NPSS patients exhibit cold hyperalgesia on the contralesional side compared to the ipsilesional side, not seen in CPSP patients (p = 0.011). A gradient-boosting approach to predicting CPSP from QST patterns before pain onset had an overall accuracy of 84.6%, with a recall and precision of 75%. Notably, both in the acute and the chronic phase, approximately 80% of CPSP and NPSS patients showed bilateral QST abnormalities.
Interpretation: Cold perception differences between CPSP and NPSS patients appear early post stroke before pain onset. Prediction of CPSP through QST patterns seems feasible. ANN NEUROL 2025;97:507-520.
© 2024 The Author(s). Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Nothing to report.
Figures
Similar articles
-
Chronic sensory stroke with and without central pain is associated with bilaterally distributed sensory abnormalities as detected by quantitative sensory testing.Pain. 2016 Jan;157(1):194-202. doi: 10.1097/j.pain.0000000000000354. Pain. 2016. PMID: 26397931
-
A study of clinical, magnetic resonance imaging, and somatosensory-evoked potential in central post-stroke pain.J Pain. 2008 Dec;9(12):1116-22. doi: 10.1016/j.jpain.2008.06.013. Epub 2008 Oct 10. J Pain. 2008. PMID: 18848810 Clinical Trial.
-
Allodynia in patients with post-stroke central pain (CPSP) studied by statistical quantitative sensory testing within individuals.Pain. 2004 Jun;109(3):357-366. doi: 10.1016/j.pain.2004.02.002. Pain. 2004. PMID: 15157697
-
Post-stroke pain case study: clinical characteristics, therapeutic options and long-term follow-up.Eur J Neurol. 2004 Apr;11 Suppl 1:22-30. doi: 10.1111/j.1471-0552.2004.00793.x. Eur J Neurol. 2004. PMID: 15061821 Review.
-
Quantitative Sensory Testing in Spinal Cord Stimulation: A Narrative Review.Neuromodulation. 2024 Aug;27(6):1026-1034. doi: 10.1016/j.neurom.2024.03.005. Epub 2024 Apr 18. Neuromodulation. 2024. PMID: 38639705 Review.
Cited by
-
Somatosensory Profile of Central Post Stroke Pain of Thalamic Origin: Findings of a Quantitative Sensory Testing Study.Eur J Pain. 2025 Sep;29(8):e70104. doi: 10.1002/ejp.70104. Eur J Pain. 2025. PMID: 40814914 Free PMC article.
References
-
- Dejerine J, Roussy G. Le syndrome thamique. Rev Neurol 1906;14:521–532.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

