Replication kinetics, pathogenicity and virus-induced cellular responses of cattle-origin influenza A(H5N1) isolates from Texas, United States
- PMID: 39727152
- PMCID: PMC11721806
- DOI: 10.1080/22221751.2024.2447614
Replication kinetics, pathogenicity and virus-induced cellular responses of cattle-origin influenza A(H5N1) isolates from Texas, United States
Abstract
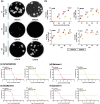
The host range of HPAIV H5N1 was recently expanded to include ruminants, particularly dairy cattle in the United States (US). Shortly after, human H5N1 infection was reported in a dairy worker in Texas following exposure to infected cattle. Herein, we rescued the cattle-origin influenza A/bovine/Texas/24-029328-02/2024(H5N1, rHPbTX) and A/Texas/37/2024(H5N1, rHPhTX) viruses, identified in dairy cattle and human, respectively, and their low pathogenic forms, rLPbTX and rLPhTX, with monobasic HA cleavage sites. Intriguingly, rHPhTX replicated more efficiently than rHPbTX in mammalian and avian cells. Still, variations in the PA and NA proteins didn't affect their antiviral susceptibility to PA and NA inhibitors. Unlike rHPbTX and rLPbTX, both rHPhTX and rLPhTX exhibited higher pathogenicity and efficient replication in infected C57BL/6J mice. The lungs of rHPhTX-infected mice produced higher inflammatory cytokines/chemokines than rHPbTX-infected mice. Our results highlight the potential risk of HPAIV H5N1 virus adaptation in human and/or dairy cattle during the current multistate/multispecies outbreak in the US.
Keywords: Cattle H5N1 virus; HPAIV; adaptation; pathogenicity; zoonosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
