DNA polymerase zeta can efficiently replicate structures formed by AT/TA repeat sequences and prevent their deletion
- PMID: 39727171
- PMCID: PMC11797062
- DOI: 10.1093/nar/gkae1254
DNA polymerase zeta can efficiently replicate structures formed by AT/TA repeat sequences and prevent their deletion
Abstract
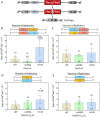
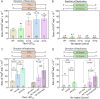
Long AT repeat tracts form non-B DNA structures that stall DNA replication and cause chromosomal breakage. AT repeats are abundant in human common fragile sites (CFSs), genomic regions that undergo breakage under replication stress. Using an in vivo yeast model system containing AT-rich repetitive elements from human CFS FRA16D, we find that DNA polymerase zeta (Pol ζ) is required to prevent breakage and subsequent deletions at hairpin and cruciform forming (AT/TA)n sequences, with little to no role at an (A/T)28 repeat or a control non-structure forming sequence. DNA polymerase eta is not protective for deletions at AT-rich structures, while DNA polymerase delta is protective, but not in a repeat-specific manner. Using purified replicative holoenzymes in vitro, we show that hairpin structures are most inhibitory to yeast DNA polymerase epsilon, whereas yeast and human Pol ζ efficiently synthesize these regions in a stepwise manner. A requirement for the Rev1 protein and the modifiable lysine 164 of proliferating cell nuclear antigen to prevent deletions at AT/TA repeats suggests a mechanism for Pol ζ recruitment. Our results reveal a novel role for Pol ζ in replicating through AT-rich hairpins and suggest a role for Pol ζ in rescue of stalled replication forks caused by DNA structures.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures






Similar articles
-
DNA polymerases eta and kappa exchange with the polymerase delta holoenzyme to complete common fragile site synthesis.DNA Repair (Amst). 2017 Sep;57:1-11. doi: 10.1016/j.dnarep.2017.05.006. Epub 2017 Jun 3. DNA Repair (Amst). 2017. PMID: 28605669 Free PMC article.
-
A novel variant of DNA polymerase ζ, Rev3ΔC, highlights differential regulation of Pol32 as a subunit of polymerase δ versus ζ in Saccharomyces cerevisiae.DNA Repair (Amst). 2014 Dec;24:138-149. doi: 10.1016/j.dnarep.2014.04.013. Epub 2014 May 10. DNA Repair (Amst). 2014. PMID: 24819597 Free PMC article.
-
Translesion polymerase eta both facilitates DNA replication and promotes increased human genetic variation at common fragile sites.Proc Natl Acad Sci U S A. 2021 Nov 30;118(48):e2106477118. doi: 10.1073/pnas.2106477118. Proc Natl Acad Sci U S A. 2021. PMID: 34815340 Free PMC article.
-
DNA polymerase ζ in DNA replication and repair.Nucleic Acids Res. 2019 Sep 19;47(16):8348-8361. doi: 10.1093/nar/gkz705. Nucleic Acids Res. 2019. PMID: 31410467 Free PMC article. Review.
-
Eukaryotic DNA polymerase ζ.DNA Repair (Amst). 2015 May;29:47-55. doi: 10.1016/j.dnarep.2015.02.012. Epub 2015 Feb 19. DNA Repair (Amst). 2015. PMID: 25737057 Free PMC article. Review.
Cited by
-
Replicative DNA polymerase epsilon and delta holoenzymes show wide-ranging inhibition at G-quadruplexes in the human genome.Nucleic Acids Res. 2025 Apr 22;53(8):gkaf352. doi: 10.1093/nar/gkaf352. Nucleic Acids Res. 2025. PMID: 40298112 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

