Uncovering topologically associating domains from three-dimensional genome maps with TADGATE
- PMID: 39727192
- PMCID: PMC11879124
- DOI: 10.1093/nar/gkae1267
Uncovering topologically associating domains from three-dimensional genome maps with TADGATE
Abstract
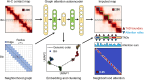
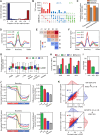
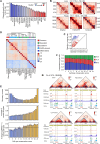
Topologically associating domains (TADs) are essential components of three-dimensional (3D) genome organization and significantly influence gene transcription regulation. However, accurately identifying TADs from sparse chromatin contact maps and exploring the structural and functional elements within TADs remain challenging. To this end, we develop TADGATE, a graph attention auto-encoder that can generate imputed maps from sparse Hi-C contact maps while adaptively preserving or enhancing the underlying topological structures, thereby facilitating TAD identification. TADGATE captures specific attention patterns with two types of units within TADs and demonstrates TAD organization relates to chromatin compartmentalization with diverse biological properties. We identify many structural and functional elements within TADs, with their abundance reflecting the overall properties of these domains. We applied TADGATE to sparse and noisy Hi-C contact maps from 21 human tissues or cell lines. That improved the clarity of TAD structures, allowing us to investigate conserved and cell-type-specific boundaries and uncover cell-type-specific transcriptional regulatory mechanisms associated with topological domains. We also demonstrated TADGATE's capability to fill in sparse single-cell Hi-C contact maps and identify TAD-like domains within them, revealing the specific domain boundaries with distinct heterogeneity and the shared backbone boundaries characterized by strong CTCF enrichment and high gene expression levels.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Dekker J., Rippe K., Dekker M., Kleckner N.. Capturing chromosome conformation. Science. 2002; 295:1306–1311. - PubMed
-
- Dostie J., Richmond T.A., Arnaout R.A., Selzer R.R., Lee W.L., Honan T.A., Rubio E.D., Krumm A., Lamb J., Nusbaum C.et al. .. Chromosome conformation capture carbon copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res. 2006; 16:1299–1309. - PMC - PubMed
-
- Simonis M., Klous P., Splinter E., Moshkin Y., Willemsen R., de Wit E., van Steensel B., de Laat W.. Nuclear organization of active and inactive chromatin domains uncovered by chromosome conformation capture-on-chip (4C). Nat. Genet. 2006; 38:1348–1354. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

