A transcription network underlies the dual genomic coordination of mitochondrial biogenesis
- PMID: 39727307
- PMCID: PMC11677238
- DOI: 10.7554/eLife.96536
A transcription network underlies the dual genomic coordination of mitochondrial biogenesis
Abstract
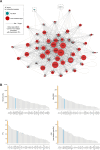
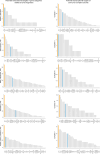
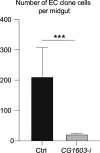
Mitochondrial biogenesis requires the expression of genes encoded by both the nuclear and mitochondrial genomes. However, aside from a handful transcription factors regulating specific subsets of mitochondrial genes, the overall architecture of the transcriptional control of mitochondrial biogenesis remains to be elucidated. The mechanisms coordinating these two genomes are largely unknown. We performed a targeted RNAi screen in developing eyes with reduced mitochondrial DNA content, anticipating a synergistic disruption of tissue development due to impaired mitochondrial biogenesis and mitochondrial DNA (mtDNA) deficiency. Among 638 transcription factors annotated in the Drosophila genome, 77 were identified as potential regulators of mitochondrial biogenesis. Utilizing published ChIP-seq data of positive hits, we constructed a regulatory network revealing the logic of the transcription regulation of mitochondrial biogenesis. Multiple transcription factors in core layers had extensive connections, collectively governing the expression of nearly all mitochondrial genes, whereas factors sitting on the top layer may respond to cellular cues to modulate mitochondrial biogenesis through the underlying network. CG1603, a core component of the network, was found to be indispensable for the expression of most nuclear mitochondrial genes, including those required for mtDNA maintenance and gene expression, thus coordinating nuclear genome and mtDNA activities in mitochondrial biogenesis. Additional genetic analyses validated YL-1, a transcription factor upstream of CG1603 in the network, as a regulator controlling CG1603 expression and mitochondrial biogenesis.
Keywords: ChIP-seq; D. melanogaster; RNA-seq; SDHA; TFAM; genetics; genomics; mitochondrial biogenesis; transcription factors.
Conflict of interest statement
FZ, AL, AF, JH, ZW, SG, ZC, HX No competing interests declared
Figures




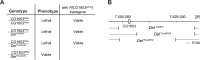





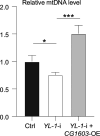
Update of
-
A transcription network underlies the dual genomic coordination of mitochondrial biogenesis.bioRxiv [Preprint]. 2024 Sep 16:2024.01.25.577217. doi: 10.1101/2024.01.25.577217. bioRxiv. 2024. Update in: Elife. 2024 Dec 27;13:RP96536. doi: 10.7554/eLife.96536. PMID: 38410491 Free PMC article. Updated. Preprint.
Similar articles
-
A transcription network underlies the dual genomic coordination of mitochondrial biogenesis.bioRxiv [Preprint]. 2024 Sep 16:2024.01.25.577217. doi: 10.1101/2024.01.25.577217. bioRxiv. 2024. Update in: Elife. 2024 Dec 27;13:RP96536. doi: 10.7554/eLife.96536. PMID: 38410491 Free PMC article. Updated. Preprint.
-
Drosophila nuclear factor DREF regulates the expression of the mitochondrial DNA helicase and mitochondrial transcription factor B2 but not the mitochondrial translation factor B1.Biochim Biophys Acta. 2013 Oct;1829(10):1136-46. doi: 10.1016/j.bbagrm.2013.07.006. Epub 2013 Jul 31. Biochim Biophys Acta. 2013. PMID: 23916463 Free PMC article.
-
Regulation of mitochondrial genome replication by hypoxia: The role of DNA oxidation in D-loop region.Free Radic Biol Med. 2016 Jul;96:78-88. doi: 10.1016/j.freeradbiomed.2016.04.011. Epub 2016 Apr 25. Free Radic Biol Med. 2016. PMID: 27091693 Free PMC article.
-
Starting the engine of the powerhouse: mitochondrial transcription and beyond.Biol Chem. 2022 Mar 31;403(8-9):779-805. doi: 10.1515/hsz-2021-0416. Print 2022 Jul 26. Biol Chem. 2022. PMID: 35355496 Review.
-
Animal models of mitochondrial DNA transactions in disease and ageing.Exp Gerontol. 2010 Aug;45(7-8):489-502. doi: 10.1016/j.exger.2010.01.019. Epub 2010 Feb 1. Exp Gerontol. 2010. PMID: 20123011 Free PMC article. Review.
Cited by
-
Mitochondria-Nuclear Crosstalk: Orchestrating mtDNA Maintenance.Environ Mol Mutagen. 2025 Jun;66(5):222-242. doi: 10.1002/em.70013. Epub 2025 May 26. Environ Mol Mutagen. 2025. PMID: 40418056 Free PMC article. Review.
-
Scalable production and purification of engineered ARRDC1-mediated microvesicles in a HEK293 suspension cell system.Sci Rep. 2025 Mar 1;15(1):7299. doi: 10.1038/s41598-025-87674-5. Sci Rep. 2025. PMID: 40025043 Free PMC article.
-
Mitochondrial DNA removal is essential for sperm development and activity.EMBO J. 2025 Mar;44(6):1749-1773. doi: 10.1038/s44318-025-00377-5. Epub 2025 Feb 11. EMBO J. 2025. PMID: 39934414 Free PMC article.
References
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

