Loss of mucin 2 and MHC II molecules causes rare resistance to murine RV infection
- PMID: 39727412
- PMCID: PMC11852729
- DOI: 10.1128/jvi.01507-24
Loss of mucin 2 and MHC II molecules causes rare resistance to murine RV infection
Abstract
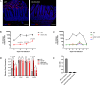
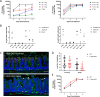
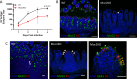
Enteric pathogen rotavirus (RV) primarily infects mature enterocytes at the tips of the intestinal villi; however, the role of secretory Paneth and goblet cells in RV pathogenesis remains unappreciated. Atoh1 knockout mice (Atoh1cKO) were used to conditionally delete Paneth, goblet, and enteroendocrine cells in the epithelium to investigate the role of secretory cells in RV infection. Unexpectedly, the number of infected enterocytes and the amount of RV shedding in the stool were greatly decreased following secretory cell deletion. Resistance to RV infection persisted for 7 days after virus inoculation, and Atoh1 knockout mice co-housed with infected wild-type mice were uninfected, based on lack of shedding virus, despite the highly infectious nature of RV. This response was directly proportional to the extent of secretory cell deletion, with infection predominantly occurring in areas containing intact secretory cells. RV infection of Muc2 knockout mice recapitulated the secretory cell deletion phenotype, indicating that goblet cell loss is responsible for attenuated infection. Transcriptome analysis of Atoh1cKO intestine via single-cell RNA sequencing revealed downregulation of MHC II molecules specifically in tip enterocytes, and MHC II-/- mice were likewise resistant to RV infection. These data suggest a previously unknown role for both MUC2 and MHC II expression in susceptibility to RV infection.IMPORTANCERotavirus (RV) is a highly contagious pathogen that primarily infects mature intestinal enterocytes. Murine rotavirus readily infects infant and adult mice, enabling evaluation of RV infection and immunity. We report that mice lacking secretory cells are one of the few genetically modified mouse lines not susceptible to murine rotavirus. Further investigation revealed loss of mucin 2 (MUC2) expression or major histocompatibility complex II (MCH II) expression recapitulated this rare resistance to rotavirus infection, suggesting a previously unrecognized link between secretory cell products and major histocompatibility complex II expression. Furthermore, these mouse models provide a platform to investigate rotavirus pathogenesis.
Keywords: Atoh1; MHC II; Muc2; RV; pathogenesis; receptor; resistance; secretory cell; tropism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Paneth cells inhibit intestinal stem cell proliferation through the bone morphogenic protein 7 pathway under rotavirus-mediated intestinal injury.World J Gastroenterol. 2025 Jul 14;31(26):107044. doi: 10.3748/wjg.v31.i26.107044. World J Gastroenterol. 2025. PMID: 40678707 Free PMC article.
-
Rotavirus NSP1 Contributes to Intestinal Viral Replication, Pathogenesis, and Transmission.mBio. 2021 Dec 21;12(6):e0320821. doi: 10.1128/mBio.03208-21. Epub 2021 Dec 14. mBio. 2021. PMID: 34903043 Free PMC article.
-
Homeostasis and function of goblet cells during rotavirus infection in mice.Virology. 2005 Jul 5;337(2):210-21. doi: 10.1016/j.virol.2005.03.039. Virology. 2005. PMID: 15882887
-
A viral enterotoxin. A new mechanism of virus-induced pathogenesis.Adv Exp Med Biol. 1999;473:73-82. Adv Exp Med Biol. 1999. PMID: 10659345 Review.
-
Rotavirus Interactions With Host Intestinal Epithelial Cells.Front Immunol. 2021 Dec 22;12:793841. doi: 10.3389/fimmu.2021.793841. eCollection 2021. Front Immunol. 2021. PMID: 35003114 Free PMC article. Review.
References
-
- Estes M, Greenberg HB. 2013. Edited by Knipe D. and Howley P. M.. Field’s virology, p 1347–1401. Lippincott Williams & Wilkins.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

