Inorganic polyphosphate and the stringent response coordinately control cell division and cell morphology in Escherichia coli
- PMID: 39727417
- PMCID: PMC11796413
- DOI: 10.1128/mbio.03511-24
Inorganic polyphosphate and the stringent response coordinately control cell division and cell morphology in Escherichia coli
Abstract
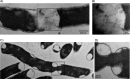
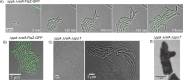
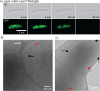
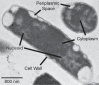
Bacteria encounter numerous stressors in their constantly changing environments and have evolved many methods to deal with stressors quickly and effectively. One well-known and broadly conserved stress response in bacteria is the stringent response, mediated by the alarmone (p)ppGpp. (p)ppGpp is produced in response to amino acid starvation and other nutrient limitations and stresses and regulates both the activity of proteins and expression of genes. Escherichia coli also makes inorganic polyphosphate (polyP), an ancient molecule evolutionary conserved across most bacteria and other cells, in response to a variety of stress conditions, including amino acid starvation. PolyP can act as an energy and phosphate storage pool, metal chelator, regulatory signal, and chaperone, among other functions. Here we report that E. coli lacking both (p)ppGpp and polyP have a complex phenotype indicating previously unknown overlapping roles for (p)ppGpp and polyP in regulating cell division, cell morphology, and metabolism. Disruption of either (p)ppGpp or polyP synthesis led to the formation of filamentous cells, but simultaneous disruption of both pathways resulted in cells with heterogenous cell morphologies, including highly branched cells, severely mislocalized Z-rings, and cells containing substantial void spaces. These mutants also failed to grow when nutrients were limited, even when amino acids were added. These results provide new insights into the relationship between polyP synthesis and the stringent response in bacteria and point toward their having a joint role in controlling metabolism, cell division, and cell growth.IMPORTANCECell division is a fundamental biological process, and the mechanisms that control it in Escherichia coli have been the subject of intense research scrutiny for many decades. Similarly, both the (p)ppGpp-dependent stringent response and inorganic polyphosphate (polyP) synthesis are well-studied, evolutionarily ancient, and widely conserved pathways in diverse bacteria. Our results indicate that these systems, normally studied as stress-response mechanisms, play a coordinated and novel role in regulating cell division, morphology, and metabolism even under non-stress conditions.
Keywords: (p)ppGpp; cell division; cell morphology; polyphosphate; stress response; stringent response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Update of
-
Inorganic polyphosphate and the stringent response coordinately control cell division and cell morphology in Escherichia coli.bioRxiv [Preprint]. 2024 Sep 12:2024.09.11.612536. doi: 10.1101/2024.09.11.612536. bioRxiv. 2024. Update in: mBio. 2025 Feb 05;16(2):e0351124. doi: 10.1128/mbio.03511-24. PMID: 39314361 Free PMC article. Updated. Preprint.
References
-
- Jain V, Kumar M, Chatterji D. 2006. ppGpp: stringent response and survival. J Microbiol 44:1–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

