A bacterial membrane-disrupting protein stimulates animal metamorphosis
- PMID: 39727418
- PMCID: PMC11796346
- DOI: 10.1128/mbio.03573-24
A bacterial membrane-disrupting protein stimulates animal metamorphosis
Abstract
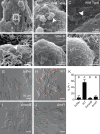
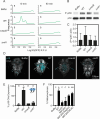
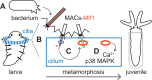
Diverse marine animals undergo a metamorphic larval-to-juvenile transition in response to surface-bound bacteria. Although this host-microbe interaction is critical to establishing and maintaining marine animal populations, the functional activity of bacterial products and how they activate the host's metamorphosis program has not yet been defined for any animal. The marine bacterium Pseudoalteromonas luteoviolacea stimulates the metamorphosis of a tubeworm called Hydroides elegans by producing a molecular syringe called metamorphosis-associated contractile structures (MACs). MACs stimulate metamorphosis by injecting a protein effector termed metamorphosis-inducing factor 1 (Mif1) into tubeworm larvae. Here, we show that MACs bind to tubeworm cilia and form visible pores on the cilia membrane surface, which are smaller and less numerous in the absence of Mif1. In vitro, Mif1 associates with eukaryotic lipid membranes and possesses phospholipase activity. MACs can also deliver Mif1 to human cell lines and cause parallel phenotypes, including cell surface binding, membrane disruption, calcium flux, and mitogen-activated protein kinase activation. Finally, MACs can also stimulate metamorphosis by delivering two unrelated membrane-disrupting proteins, MLKL and RegIIIɑ. Our findings demonstrate that membrane disruption by MACs and Mif1 is necessary for Hydroides metamorphosis, connecting the activity of a bacterial protein effector to the developmental transition of a marine animal.
Importance: This research describes a mechanism wherein a bacterium prompts the metamorphic development of an animal from larva to juvenile form by injecting a protein that disrupts membranes in the larval cilia. Specifically, results show that a bacterial contractile injection system and the protein effector it injects form pores in larval cilia, influencing critical signaling pathways like mitogen-activated protein kinase and calcium flux, ultimately driving animal metamorphosis. This discovery sheds light on how a bacterial protein effector exerts its activity through membrane disruption, a phenomenon observed in various bacterial toxins affecting cellular functions, and elicits a developmental response. This work reveals a potential strategy used by marine organisms to respond to microbial cues, which could inform efforts in coral reef restoration and biofouling prevention. The study's insights into metamorphosis-associated contractile structures' delivery of protein effectors to specific anatomical locations highlight prospects for future biomedical and environmental applications.
Keywords: cilia; contractile injection system; effector; metamorphosis; pore-forming toxin; secretion systems; toxin.
Conflict of interest statement
K.E.M., T.L.D., and N.J.S. are co-inventors on provisional U.S. patent application entitled "Protein and Peptide Delivery Systems and Methods for Using Them with Human Cells" and assigned to San Diego State University Research Foundation.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

