Molecular Pathophysiology of Chronic Thromboembolic Pulmonary Hypertension: A Clinical Update from a Basic Research Perspective
- PMID: 39727495
- PMCID: PMC11673787
- DOI: 10.3390/arm92060044
Molecular Pathophysiology of Chronic Thromboembolic Pulmonary Hypertension: A Clinical Update from a Basic Research Perspective
Abstract
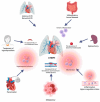
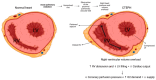
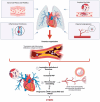
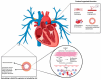
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare but severe condition characterized by persistent obstruction and vascular remodeling in the pulmonary arteries following an acute pulmonary embolism (APE). Although APE is a significant risk factor, up to 25% of CTEPH cases occur without a history of APE or deep vein thrombosis, complicating the understanding of its pathogenesis. Herein, we carried out a narrative review discussing the mechanisms involved in CTEPH development, including fibrotic thrombus formation, pulmonary vascular remodeling, and abnormal angiogenesis, leading to elevated pulmonary vascular resistance and right heart failure. We also outlined how the disease's pathophysiology reveals both proximal and distal pulmonary artery obstruction, contributing to the development of pulmonary hypertension. We depicted the risk factors predicting CTEPH, including thrombotic history, hemostatic disorders, and certain medical conditions. We finally looked at the molecular mechanisms behind the role of endothelial dysfunction, gene expression alterations, and inflammatory processes in CTEPH progression and detection. Despite these insights, there is still a need for improved diagnostic tools, biomarkers, and therapeutic strategies to enhance early detection and management of CTEPH, ultimately aiming to reduce diagnostic delay and improve patient outcomes.
Keywords: acute pulmonary embolism; chronic thromboembolic pulmonary hypertension; fibrotic thrombus formation; gene expression; pulmonary hypertension; pulmonary vascular remodeling.
Conflict of interest statement
The authors declare no conflicts of interest regarding the revision and publication of this manuscript.
Figures
Similar articles
-
[From acute pulmonary embolism to chronic thromboembolic pulmonary hypertension: Pathobiology and pathophysiology].Arch Cardiol Mex. 2017 Jan-Mar;87(1):26-34. doi: 10.1016/j.acmx.2016.10.003. Epub 2016 Dec 10. Arch Cardiol Mex. 2017. PMID: 27956338 Review. Spanish.
-
Why acute pulmonary embolism becomes chronic thromboembolic pulmonary hypertension: clinical and genetic insights.Curr Opin Pulm Med. 2013 Sep;19(5):422-9. doi: 10.1097/MCP.0b013e328364379f. Curr Opin Pulm Med. 2013. PMID: 23907454 Review.
-
Crosstalk between endothelial cell and thrombus in chronic thromboembolic pulmonary hypertension: perspective.Histol Histopathol. 2013 Feb;28(2):185-93. doi: 10.14670/HH-28.185. Histol Histopathol. 2013. PMID: 23275302 Review.
-
Update on chronic thromboembolic pulmonary hypertension.Trends Cardiovasc Med. 2017 Jan;27(1):29-37. doi: 10.1016/j.tcm.2016.05.010. Epub 2016 May 25. Trends Cardiovasc Med. 2017. PMID: 27345156 Review.
-
Early return of reflected waves increases right ventricular wall stress in chronic thromboembolic pulmonary hypertension.Am J Physiol Heart Circ Physiol. 2020 Dec 1;319(6):H1438-H1450. doi: 10.1152/ajpheart.00442.2020. Epub 2020 Oct 9. Am J Physiol Heart Circ Physiol. 2020. PMID: 33035435
References
-
- Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., Huisman M.V., Humbert M., Jennings C.S., Jimenez D., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur. Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. - DOI - PubMed
-
- Gonzalez-Hermosillo L.M., Cueto-Robledo G., Navarro-Vergara D.I., Garcia-Cesar M., Torres-Rojas M.B., Graniel-Palafox L.E., Castro-Escalante K.Y., Castro-Diaz A.M. Post-pulmonary embolism syndrome: A reminder for clinicians. Asian Cardiovasc. Thorac. Ann. 2024;32:336–344. doi: 10.1177/02184923241272913. - DOI - PubMed
-
- Ende-Verhaar Y.M., Cannegieter S.C., Vonk Noordegraaf A., Delcroix M., Pruszczyk P., Mairuhu A.T., Huisman M.V., Klok F.A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: A contemporary view of the published literature. Eur. Respir. J. 2017;49:1601792. doi: 10.1183/13993003.01792-2016. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

