Successful Therapy over 12 Months of People with Cystic Fibrosis with Rare Non-phe508del Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Mutations with Elexacaftor/Tezacaftor/Ivacaftor (ETI)
- PMID: 39727500
- PMCID: PMC11672886
- DOI: 10.3390/arm92060049
Successful Therapy over 12 Months of People with Cystic Fibrosis with Rare Non-phe508del Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Mutations with Elexacaftor/Tezacaftor/Ivacaftor (ETI)
Abstract
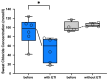
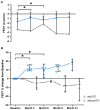
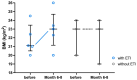
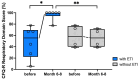
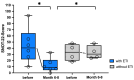
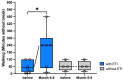
Background: Elexacaftor/Tezacaftor/Ivacaftor (ETI) is a CFTR modulator therapy approved for people with cystic fibrosis (pwCF) who have at least one phe508del mutation. However, its approval in the European Union (EU) for pwCF with non-phe508del mutations is lacking, because data on treatment response in this subgroup are scarce. Methods: This retrospective observational study evaluated six pwCF (ages 6 to 66) with responsive CFTR mutations (M1101K, R347P, 2789+5G>A, G551D) undergoing off-label ETI therapy. Evaluations were conducted at 0, 3, 6, 9, and 12 months, assessing lung function (FEV1), sweat chloride levels, body mass index (BMI), quality of life, medication satisfaction, ear, nose and throat (ENT) symptoms, and physical activity. A control group of four pwCF with classic symptoms and no ETI treatment was included. Results: FEV1 improved significantly after 3 and 6 months (p < 0.05) and stabilized by 12 months. Sweat chloride levels decreased significantly, with four pwCF achieving levels <60 mmol/L. Improvements in the upper and lower airway symptoms, medication satisfaction, and increased BMI were noted. Conclusions: ETI demonstrates high efficacy in this small group of pwCF with rare CFTR mutations, offering a treatment option that warrants further monitoring and evaluation.
Keywords: cystic fibrosis; elexacaftor/tezacaftor/ivacaftor; non-phe508del CFTR mutations; off-label use; rare CFTR mutations.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2020 Dec 17;12(12):CD010966. doi: 10.1002/14651858.CD010966.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2023 Nov 20;11:CD010966. doi: 10.1002/14651858.CD010966.pub4. PMID: 33331662 Free PMC article. Updated.
-
Real-world effectiveness and safety of elexacaftor/tezacaftor/ivacaftor in cystic fibrosis patients with Phe508del -gating and -residual function genotypes.Respir Med. 2025 Aug-Sep;245:108221. doi: 10.1016/j.rmed.2025.108221. Epub 2025 Jun 24. Respir Med. 2025. PMID: 40571167
-
The expanded French compassionate programme for elexacaftor-tezacaftor-ivacaftor use in people with cystic fibrosis without a F508del CFTR variant: a real-world study.Lancet Respir Med. 2024 Nov;12(11):888-900. doi: 10.1016/S2213-2600(24)00208-X. Epub 2024 Aug 13. Lancet Respir Med. 2024. PMID: 39151434
-
VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles.N Engl J Med. 2018 Oct 25;379(17):1612-1620. doi: 10.1056/NEJMoa1807120. Epub 2018 Oct 18. N Engl J Med. 2018. PMID: 30334692 Free PMC article. Clinical Trial.
-
The clinical effectiveness of elexacaftor/tezacaftor/ivacaftor (ETI) for people with CF without a F508del variant: A systematic review and meta-analysis.J Cyst Fibros. 2024 Sep;23(5):950-958. doi: 10.1016/j.jcf.2024.07.012. Epub 2024 Jul 23. J Cyst Fibros. 2024. PMID: 39048464
References
-
- Nährlich L., Burkhart M., Wosniok J. German Cystic Fibrosis Registry Annual Report. 2022. [(accessed on 8 July 2024)]. Available online: https://www.muko.info/fileadmin/user_upload/was_wir_tun/register/bericht....
-
- Veit G., Roldan A., Hancock M.A., Da Fonte D.F., Xu H., Hussein M., Frenkiel S., Matouk E., Velkov T., Lukacs G.L. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight. 2020;5:e139983. doi: 10.1172/jci.insight.139983. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

