Potential Unlocking of Biological Activity of Caffeic Acid by Incorporation into Hydrophilic Gels
- PMID: 39727552
- PMCID: PMC11675749
- DOI: 10.3390/gels10120794
Potential Unlocking of Biological Activity of Caffeic Acid by Incorporation into Hydrophilic Gels
Abstract
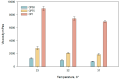
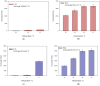
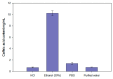
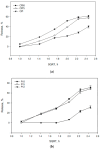
Caffeic acid, a phenolic compound with antioxidant and antimicrobial properties, shows promise in the dermatological field. The research aimed to incorporate caffeic acid into hydrophilic gels based on poloxamer 407, carbomer 980, and their mixture in order to enhance its biological activity. Different gel formulations were prepared using different concentrations of these polymers to optimize caffeic acid release characteristics. The results showed that increasing the concentration of polymeric materials increased the viscosity and slowed down the release of caffeic acid. The antioxidant and antimicrobial activities of the gels were assessed. The results confirmed the potential of hydrophilic gels as delivery systems for caffeic acid, with formulations showing antimicrobial activity against Gram-positive Staphylococcus aureus bacteria and antifungal activity against Candida albicans fungus. This study provides a perception of the development of new semi-solid caffeic acid-based formulations for therapeutic and cosmetic applications.
Keywords: antimicrobial; caffeic acid; carbomer; hydrogel; poloxamer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Ravetti S., Clemente C., Brignone S., Hergert L., Allemandi D., Palma S. Ascorbic Acid in Skin Health. Cosmetics. 2019;6:58. doi: 10.3390/cosmetics6040058. - DOI
-
- Kabała-Dzik A., Rzepecka-Stojko A., Kubina R., Jastrzębska-Stojko Ż., Stojko R., Wojtyczka R.D., Stojko J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules. 2017;22:1554. doi: 10.3390/molecules22091554. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

